Abstract
Purpose. This study investigated the impact of early tumor shrinkage (ETS) on progression-free- (PFS) and overall survival (OS) in patients with metastatic colorectal cancer (mCRC) treated within the AIO KRK 0104 trial as first-line therapy. Moreover, correlations of ETS with clinical characteristics and prognostic markers were evaluated. Patients and methods. In total, 121 patients were included into this analysis. Patients were treated with cetuximab combined with either CAPIRI or CAPOX. ETS at six weeks was defined as a relative change of ≥ 20% in the sum of the longest diameters of target lesions compared to baseline. Survival times were compared between patients with ETS ≥ 20% versus no-ETS. Results. ETS ≥ 20% was observed in 59% of all patients with KRAS wild-type tumors. In these patients ETS ≥ 20% was associated with higher overall response rate (82% vs. 19%, p < 0.001). Also, PFS (8.9 vs. 4.7 months, p < 0.001) and OS (31.6 vs. 15.8 months, p = 0.005) were significantly superior in ETS ≥ 20% of patients compared to no-ETS. In patients with KRAS mutant mCRC ETS ≥ 20% neither had an effect on PFS nor OS. Cetuximab-induced skin toxicity correlated with the occurrence of ETS ≥ 20% (p = 0.002). Conclusion. In patients with KRAS wild-type tumors treated with cetuximab plus capecitabine-based chemotherapy ETS ≥ 20% is an important predictor of favorable outcome.
Mutations in the KRAS proto-oncogene have been identified as negative predictors of response to anti-EGFR antibodies such as cetuximab or panitumumab leading to an exclusion of affected patients from anti-EGFR therapy [Citation1–7]. Nevertheless, even in first-line trials response to anti-EGFR-based treatment is limited to less than 60% of all patients with KRAS wild-type (wt) tumors [Citation3–5]. Therefore, additional biological or clinical markers are needed to identify those patients that are most likely to benefit from anti-EGFR treatment. Recently, Piessevaux and coworkers demonstrated that early tumor shrinkage (ETS) ≥ 20% was associated with prolonged overall survival times (OS) in patients with KRAS wt tumors receiving cetuximab-based first-line therapy. They analyzed ETS ≥ 20% after treatment duration of eight weeks and selected ≥ 20% tumor reduction in diameter as a cut-off differentiating early response in shrinkers from non-shrinkers [Citation8]. In patients with KRAS wt tumors ETS ≥ 20% was associated with long-term survival, showing a significantly greater effect in patients that received chemotherapy plus cetuximab compared to patients that were treated with chemotherapy alone. This finding was underlined by significant treatment interaction testing. Therefore, the authors concluded that ETS ≥ 20% might be an indicator of cetuximab-related antitumor activity in patients with KRAS wt metastatic colorectal cancer (mCRC). Other authors demonstrated that the prognostic value of ETS in mCRC-patients receiving chemotherapy alone depends on the ETS cut-off: greater ETS was clearly associated with longer survival [Citation9].
Previously reported data from the COIN study suggested that the combination of cetuximab with capecitabine might be less effective than combinations with an infusional fluoropyrimidine [Citation10]. The present analysis therefore asks the question if ETS ≥ 20% predicts progression-free survival (PFS) and OS in patients receiving cetuximab plus capecitabine-based regimens. In the present investigation, we evaluated patients with mCRC that received cetuximab in combination with capecitabine plus oxaliplatin (CAPOX) or plus irinotecan (CAPIRI) as first-line treatment. On the one hand, ETS ≥ 20% was correlated with KRAS mutation occurring in the tumor, while on the other hand, patient-related parameters such as skin toxicity and patient characteristics were analyzed in the context of ETS-prediction.
ETS ≥ 20% represents a post-randomization marker that was assessed six weeks after the start of systemic treatment in this trial. Therefore, a potential guarantee-time bias as described by Anderson and coworkers [Citation11] was addressed by a landmark analysis.
Methods
KRAS mutation detection
KRAS testing was performed in a German reference laboratory for KRAS analysis (University of Munich, Department of Pathology). After dissection of tumor containing areas, DNA was isolated using Qiagen DNA Micro-Amp-kits. Detection of mutations in codons 12 and 13 of the KRAS proto-oncogene was then performed by pyrosequencing using Qiagen's PyroMark Gold® kits together with a Q24 pyrosequencer device. This procedure resulted in a specificity of 0.98 and sensitivity of 0.99 for the detection of mutations in the KRAS proto-oncogene [Citation12].
Study design and treatment schedule
The AIO KRK 0104 study was designed as an open-label, randomized phase II study which was conducted at 35 centers in Germany. Random assignment was centrally performed by fax. In both study arms, cetuximab was given at an initial dose of 400 mg/m2 as a 120-minute infusion, followed by weekly infusions of 250 mg/m2 over 60 minutes. Patients in arm A received chemotherapy with CAPIRI (i.e. oral capecitabine 800 mg/m2 twice daily on days 1 through 14, followed by a one-week rest period plus irinotecan 200 mg/m2 as a 30-minute intravenous infusion on day 1). In patients older than 65 years, doses were further reduced by 20%. Patients in arm B received chemotherapy with CAPOX (i.e. capecitabine 1000 mg/m2 twice daily on days 1 through 14, followed by a one-week rest period plus oxaliplatin 130 mg/m2 as a 120-minute intravenous infusion on day 1). Treatment cycles were repeated every three weeks until disease progression or unacceptable toxicity [Citation13,Citation14]. A final update on survival data was done in June 2011 [Citation14].
Patients
One hundred and twenty-one of the 146 patients that were evaluated for KRAS/BRAF mutations within the AIO KRK 0104 trial [Citation14] were included in this analysis. Twenty-five patients were not included in this analysis due to early progression/death in 10 patients and in 15 patients due to withdrawal of informed consent, serious adverse events within the first weeks of therapy or protocol violations concerning response evaluation.
End points
The present investigation was performed as an exploratory analysis. Radiological tumor response [complete remission (CR), partial remission (PR), stable disease (SD), progressive disease (PD)] was assessed according to RECIST (version 1.0) criteria. Response evaluation was performed at six-week intervals. PFS was defined as the interval between randomization and first documentation of progression or death; OS was calculated as the time between randomization and death due to any reason. Patients alive were censored at the last time point of patient contact.
ETS in this trial was defined as a reduction of ≥ 20% in diameter if a single lesion was observed. In case of more than one lesion ETS was calculated based on the sum of lesions. We evaluated ETS at six weeks when patients of the AIO KRK 0104 trial were treated with two cycles of chemotherapy plus cetuximab. An alteration of two weeks was tolerated.
Statistical analysis
Data were summarized by properly chosen measures of location and spread for continuous variables and by proportions for discrete variables. Fisher´s exact-tests and χ2-tests were applied to estimate differences in response or baseline characteristics. Differences in OS and PFS were estimated using the Kaplan-Meier method and were compared between the groups by log-rank tests. Further, differences in PFS and OS were analyzed using Cox regressions. All statistical analyses were performed with SPSS version 20, IBM, New York, USA and R (version 2.13.0; The R Foundation for Statistical Computing/R Development Core Team, GNU General Public License). The present analysis included only patients that were evaluable for ETS after six weeks of systemic treatment. This approach of a landmark analysis according to the proposition by Anderson and coworkers [Citation11] was chosen to eliminate a potential guarantee-time bias. Since no patients were ‘lost for analysis’ between start of treatment and ETS assessment this procedure excludes an impact of guarantee time bias on survival.
Results
Study population
This exploratory analysis includes 121 patients who received cetuximab plus CAPOX or cetuximab plus CAPIRI as first-line treatment of mCRC. Baseline characteristics of the whole patient population were recently published [Citation14]. Patient characteristics according to the analyzed subgroups are shown in .
Table I. Patient characteristics.
In the AIO KRK 0104 trial KRAS wt tumors seemed more frequent within the group of ETS ≥ 20% than in no-ETS patients (71% vs. 53%, p = 0.06). Favorable baseline characteristics were a little more frequent in our ETS ≥ 20% population than in the no-ETS population. These trends were seen for liver metastasis, liver limited metastasis, lymph node metastases, prior adjuvant treatment and multi organ involvement.
Cetuximab-associated skin toxicity of any grade (I–III) correlated well with ETS (100% of ETS ≥ 20% patients vs. 86% of no-ETS patients, p = 0.002). None of eight patients without any cetuximab-based skin toxicity developed ETS ≥ 20% (). If skin toxicity was correlated as grade 0–1 versus grade 2–4, significance was lost. No correlation of intensity of skin-toxicity and ETS ≥ 20% could be detected.
Impact of ETS on treatment efficacy in the AIO KRK 0104 trial
In our study cohort, ETS ≥ 20% was observed in 52% (63/121) of all patients and was associated with longer median PFS [8.6 vs. 5.1 months, p < 0.001, hazard ratio (HR): 0.50 (95% CI 0.34–0.73)] and longer median OS [28.7 vs. 17.0 months, p = 0.023, HR: 0.63 (95% CI 0.43–0.94)] ().
Table II. Parameters of treatment response.
Impact of ETS on treatment efficacy with respect to KRAS mutation
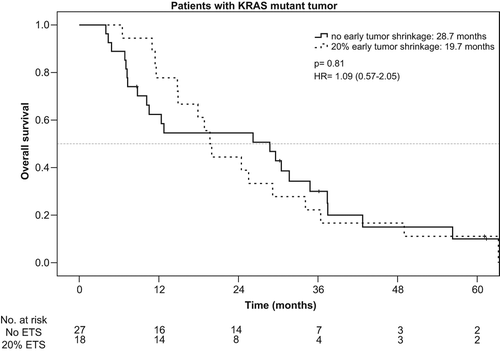
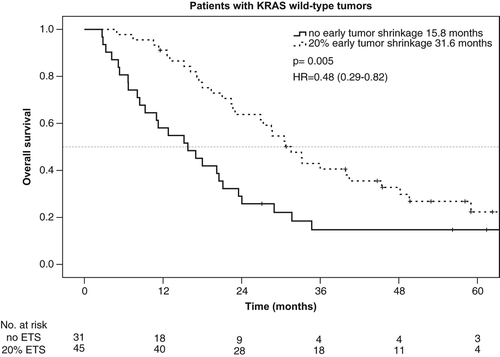
Taking KRAS mutation into account, the rate of ETS ≥ 20% was 59% (45/76) in patients with KRAS wt tumors and 40% (18/45) in patients with KRAS mutant mCRC. ETS ≥ 20% translated into a beneficial outcome only in patients with KRAS wt tumors, but not in patients bearing KRAS mutant tumors. PFS and OS in the KRAS wt-population reached 8.9 vs. 4.7 months [p < 0.001, HR: 0.37 (95% CI, 0.23–0.60)] and 31.6 vs. 15.8 months [p = 0.005, HR: 0.48 (95% CI 0.29–0.82)] in patients with ETS ≥ 20% vs. no-ETS (, and ).
Figure 1. Progression-free survival – KRAS-Wt population in the AIO KRK 0104 trial according to early tumor shrinkage.
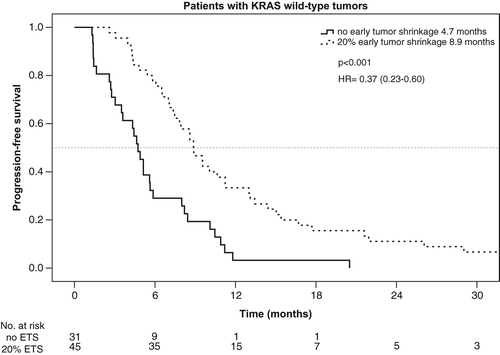
Figure 2. Overall survival – KRAS-WT population of the AIO KRK 0104 trial according to early tumor shrinkage.
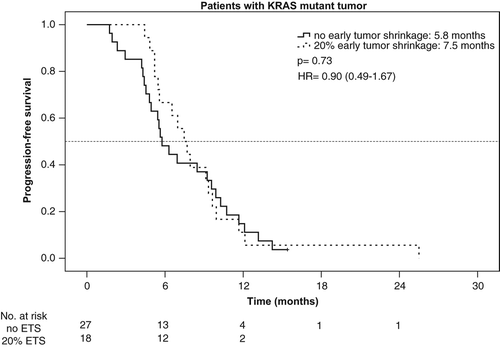
PFS and OS in patients with KRAS mutant mCRC were neither associated with significantly improved PFS [7.5 vs. 5.8 months, p = 0.73, HR: 0.90 (95% CI 0.49–1.67)], nor with longer OS (19.7 vs. 28.7 months, p = 0.81, HR: 1.09 (95% CI 0.57–2.05)] ( and ).
Discussion
KRAS mutation is an established negative predictor of treatment response to anti-EGFR agents in mCRC. Accordingly, the use of anti-EGFR antibodies such as cetuximab is limited to patients with KRAS wt tumors. However, even in first-line treatment in patients with KRAS wt mCRC, response rates following anti-EGFR-antibody-based therapy did not exceed 60% [Citation3–5] and therefore, research regarding predictive markers beyond KRAS is ongoing. Changes of the initial tumor size observed at the first reevaluation (ETS) are known prognostic parameters for outcome of patients with mCRC receiving chemotherapy and depend on the respective cut-off value [Citation9]. In patients with KRAS wt mCRC receiving cetuximab-based therapy, ETS ≥ 20% was found to correlate significantly with PFS and OS in first-line treatment [Citation8] and at a cut-off of > 10% also in pretreated patients [Citation15].
In the AIO KRK 0104 trial, 59% of patients with KRAS wt tumors developed ETS ≥ 20% at the time of first tumor-reevaluation, which was scheduled six weeks after the start of treatment. These 59% compare well with the frequencies observed within the CRYSTAL-trial-62% (FOLFIRI plus cetuximab) and the OPUS-study (FOLFOX4 plus cetuximab)-69% where eight-week intervals for reevaluation were used.
In patients with KRAS wt mCRC the differences in outcome observed in the AIO KRK 0104 trial between ETS ≥ 20% versus no-ETS appear moderate with regard to PFS (8.9 vs. 4.7 months, Δ PFS = 4.2 months, HR: 0.37) but translate into a relative prolongation of OS (31.6 vs. 15.8 months, Δ OS = 15.8 months, HR: 0.48) of similar extent. This observation compares well to the data from the OPUS- and CRYSTAL-trial concerning effects on PFS (Δ PFS = 6.8 and 6.2 months), and OS (Δ OS = 11.4 and 10.3 months) by ETS ≥ 20% in patients with KRAS wt tumors that received cetuximab-based treatment [Citation8] ().
Table III. ETS in KRAS wt-mCRC: CRYSTAL-, OPUS- and AIO KRK 0104 trial.
It is clearly of great interest to clarify the relation of ETS ≥ 20% to baseline characteristics. Concerning this question the present analysis is highly limited by its retrospective and exploratory nature based on subgroup analyses and low patient numbers. However, a positive trend might suggest that ETS ≥ 20% patients are more likely to have liver metastasis and no metastasis in lymph nodes as well as being untreated with adjuvant chemotherapy. This information on patient characteristics and ETS ≥ 20% has not been reported in other cetuximab-based first-line trials such as OPUS and CRYSTAL [Citation8].
A correlation of EGFR-antibody-related skin-toxicity and outcome has been observed in various trials [Citation5,Citation6]. Therefore, a correlation of skin-toxicity and ETS ≥ 20% as observed in our study might have been expected as both markers are known to correlate with favorable outcome in mCRC patients treated with cetuximab. In the present analysis, all patients with ETS ≥ 20% also presented skin-toxicity (grades 1–4). However, 14% of no-ETS patients had no skin toxicity (p = 0.002). These data indicate that skin toxicity does not guarantee ETS ≥ 20% but lack of skin toxicity highly correlates with no-ETS.
The prognostic/predictive role of ETS ≥ 20% in patients with mCRC that received cetuximab-based first-line therapy was only observed in patients with KRAS wt tumors in the AIO KRK 0104 trial and was not evident in patients with KRAS mutation. Our results are, of course, limited by small sample size and a biologic ratio concerning the lack of prognostic power of ETS ≥ 20% in KRAS mutant mCRC is not obvious. However, Tejpar and coworkers demonstrated a similar effect in patients with KRAS p.G13D mutant mCRC showing a significantly increased response rate undergoing cetuximab-based first-line therapies (40.5 vs. 22.0%) without substantial benefit in terms of OS (HR: 0.89) [Citation16].
In the AIO KRK 0104 trial, ETS ≥ 20% appears as an early and powerful indicator of PFS and OS. This effect was limited to patients with KRAS wt tumors. However, the correlation of ETS (evaluated by various cut-off levels) with outcome has also been observed in unselected patients treated with chemotherapy alone [Citation9]. Therefore, differences in treatment (use of anti-EGFR agents) may require different cut-offs levels for ETS (according to the individual effect of the regimens on response to therapy) to obtain optimal separation of prognostic subgroups.
While ETS ≥ 20% may clearly indicate patients benefitting from therapy, it is more difficult to draw conclusions for no-ETS patients. This is specifically important since no-ETS is not a homogeneous group, but consists of patients developing new lesions, patients progressing during treatment and patients having minor responses (ETS of 0–20%). The characterization of these subgroups requires prospectively planned evaluation in clinical trials. Since ETS ≥ 20% has proven its power as surrogate marker of favorable outcome in various cohorts, ETS could be of interest for designing clinical trials that need to separate patients into prognostic subgroups during the trial.
Acknowledgments
We thank all of the patients and their families, the participating centers of the AIO KRK 0104 trial, and the entire medical staff who contributed to patient care. We also thank Matthias Wolff for expert secretarial help and organization. The AIO KRK 0104 trial was supported by Merck.
Potential conflicts of interest: DPM: Travel support: Amgen, Merck, Roche; Honoraria: Amgen, Merck; RPL: Travel support: Merck; SS: Travel support: Merck, Roche; Honoraria: Merck, Roche; CG: Travel support: Roche; VH: Honoraria/Advisory Boards: Amgen, Merck, Roche. UM: travel support/research grant: Merck.
References
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091–6.
- Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040–8.
- Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9.
- Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol 2011;22:1535–46.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol 2010;28:4697–705.
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–64.
- Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757–65.
- Piessevaux H, van Cutsem E, Bokemeyer C, Schlichting M, Heeger S, Tejpar S. Early tumor shrinkage and clinical outcome in metastatic colorectal cancer: Analysis of predictive utility in the CRYSTAL and OPUS studies. JSMO, Osaka. 2012 July 28th.
- Suzuki C, Blomqvist L, Sundin A, Jacobsson H, Byström P, Berglund A, et al. The initial change in tumor size predicts response and survival in patients with metastatic colorectal cancer treated with combination chemotherapy. Ann Oncol 2012;23:948–54.
- Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103–14.
- Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1:710–9.
- Neumann J, Zeindl-Eberhart E, Kirchner T, Jung A. Frequency and type of KRAS mutations in routine diagnostic analysis of metastatic colorectal cancer. Pathol Res Pract 2009;205:858–62.
- Moosmann N, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK 0104—A randomized trial of the German AIO CRC Study Group. J Clin Oncol 2011;29:1050–8.
- Modest DP, Jung A, Moosmann N, Laubender RP, Giessen C, Schulz C, et al. The influence of KRAS and BRAF mutations on the efficacy of cetuximab-based first-line therapy of metastatic colorectal cancer: An analysis of the AIO KRK 0104 trial. Int J Cancer 2012;131:980–6.
- Piessevaux H, Buyse M, De Roock W, Prenen H, Schlichting M, Van Cutsem E, et al. Radiological tumor size decrease at week 6 is a potent predictor of outcome in chemorefractory metastatic colorectal cancer treated with cetuximab (BOND trial). Ann Oncol 2009;20:1375–82.
- Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol 2012;30:3570–7.