Abstract
Background. Chemoradiotherapy is an effective treatment for anal cancer, yet from follow-up many survivors seem to suffer from late effects. Data of long-term health-related quality of life (HRQOL) in anal cancer survivors are limited, and there is a growing interest in cancer survivorship. Material and methods. A national cohort of all anal cancer survivors treated with curative chemoradiotherapy in 2000–2007 was invited to a cross-sectional study. Of 199 eligible survivors, 128 (64%) returned the questionnaires, the median time since diagnosis was 66 months. The median age was 61 years and 79% were women. HRQOL was evaluated with EORTC questionnaires QLQ-C30 and QLQ-CR29, and neurotoxicity with the Scale of Chemotherapy-Induced Neurotoxicity. An age- and sex-matched reference group of volunteers (n = 269) not treated for pelvic cancer answered the same questionnaires. Results from QLQ-C30 of the reference group were compared to Norwegian and Dutch normative data. Results. The mean scores of anal cancer survivors were poorer compared to volunteers and normative data. Anal cancer survivors reported significant impairment of function, especially social and role function, compared to the volunteers (difference ≥ 20 points, p < 0.001). Survivors had markedly increased scores for fatigue, dyspnoea, insomnia and diarrhoea (difference ≥ 15 points, p < 0.001). The global quality of life was significantly reduced (difference 15 points, p < 0.001). Anal cancer survivors had increased stool frequency, more buttock pain, flatulence, faecal incontinence, impotence (males), dyspareunia and reduced sexual interest (females) (difference ≥ 15 points, p < 0.001). There was increased frequency of tinnitus in survivors treated with cisplatin-based chemotherapy (p = 0.004). Conclusions. Survivors after chemoradiotherapy for anal cancer have significant long-term impairment of HRQOL. Reduced social, role and sexual function, and increased diarrhoea, incontinence for gas and stools, and buttock pain were commonly reported. Increased awareness of this may lead to better management of late effects and better care for cancer survivors.
Squamous cell carcinoma of the anal region is a rare malignancy, with an incidence of approximately 1 in 100 000; in Norway, around 50–60 patients are diagnosed per year. Sphincter preserving treatment with chemoradiotherapy (CRT) is the standard treatment [Citation1]. A recent randomised study [Citation2] has shown that radiotherapy combined with mitomycin C and 5-fluorouracil is the most effective treatment and has acceptable toxicity. We have recently shown a relatively good prognosis in a national cohort of 328 Norwegian patients treated with CRT for anal carcinoma, with a five-year cancer-specific survival (CSS) of 75% [Citation3].
This effective treatment may result in unavoidable late effects in anal cancer survivors with impact on their health-related quality of life (HRQOL). There is no gold standard method of reporting late effects. Physicians have been shown to underreport survivors’ symptoms [Citation4]. Patient-reported outcomes (PROs) are based on subjective information directly from the patient, hence focusing on important aspects of cancer survivorship from the patients’ perspective.
There is limited knowledge of the incidence and severity of late effects after CRT for anal cancer, the consequences for the patient, and how to handle late effects [Citation5–9]. Cancer survivors from an unselected, national cohort [Citation3] were invited to a follow-up study. The aim of the present study was to investigate the HRQOL in the long-term follow-up of anal cancer survivors compared with a reference group from the normal population.
Material and methods
Patients
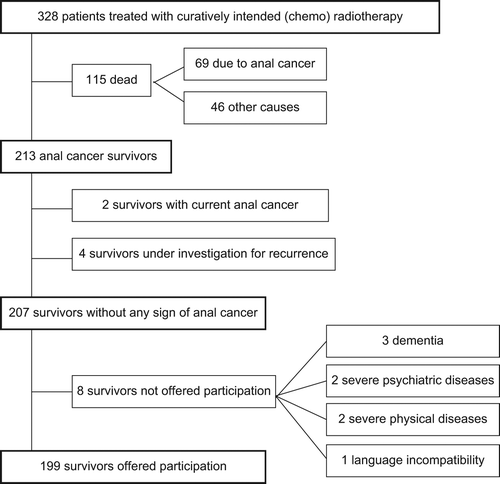
All patients diagnosed in Norway with squamous cell carcinoma of the anal region between 1 July 2000 and 30 June 2007, treated with curatively intended CRT, n = 328, were included in a study, and the treatment results on survival and recurrence have been published [Citation3]. This unselected and complete national database included characteristics of patients, tumour and treatment, and comorbidity. Survivors with a minimum follow-up of two years after diagnosis and no signs of disease recurrence, without comorbidity incompatible with participation, n = 199, were invited to participate in a cross-sectional study (). Eligible survivors received an invitation by mail between August 2009 and March 2010 from the hospital responsible for their treatment. Non-responders received up to two reminders. Survivors who signed informed consent were sent questionnaires and were contacted for telephone interview.
Volunteers
To enable better interpretation of the survivors’ responses, an age- and sex-matched group was obtained from the general population to complete the same questionnaires as the survivors. The establishment of this reference group was necessary since there were no suitable normative date available for this group of survivors and for some of the instruments used. Participants were randomly drawn from the National Population Register and invited by mail to participate. Names indicating foreign origin (n = 39) were excluded to ensure language compatibility. History of cancer in the pelvis or abdomen excluded participation. To obtain two volunteers per survivor in each sex- and age group of 10 years, a total of 1211 persons were invited. This was done by repeated random draws from the National Population Register until the required number was reached. One reminder was sent to non-responders.
Data collection and questionnaires
The pre-specified hypothesis was that survivors after anal cancer suffered from symptoms related to late effects after radiotherapy with impaired anorectal, urinary, and sexual function and more pelvic pain, resulting in reduced HRQOL. The European Organisation of Research and Treatment of Cancer (EORTC) core questionnaire (QLQ-C30) version 3.0 was used. This validated cancer-specific 30-item questionnaire [Citation10] contains five functional scales assessing physical, role, emotional, cognitive, and social function, three symptom scales assessing fatigue, nausea and vomiting, and pain, six single items assessing symptoms commonly reported by cancer patients, and a global health-status.
To our knowledge, there is no disease-specific instrument to assess HRQOL in patients with anal carcinoma. The EORTC module for colorectal cancer CR29 (QLQ-CR29) version 2.1 was used since it contains questions considered relevant to this patient group. This 29-item questionnaire incorporates four scales assessing urinary frequency, faecal seepage, stool consistency, and body image and single items including urinary incontinence, dysuria, abdominal pain, buttock pain, bloating, anxiety, flatulence, faecal incontinence, sexual interest, impotence, and dyspareunia [Citation11].
All items had response categories with four levels, from ‘not at all’ to ‘very much’, except the two items for global quality of life (QOL), which used seven-point items ranging from ‘very poor’ to ‘excellent’. A raw score was estimated by the average of the items that contributed to a scale. The score was standardised by linear transformation into a score ranging from 0–100 according to the EORTC scoring manual [Citation12]. A high score on the global QOL/functional scales represents a high QOL or a high/healthy level of functioning, and a high score for the symptom scale/items represents a high level of symptoms. A 10-point variation in the 0–100 scale has been shown to indicate a moderate clinical difference and a variation in score of > 20 points a large clinical difference [Citation13].
Some patients had been treated with cisplatin-based chemotherapy. No gold standard exists to explore the incidence and severity of neurotoxicity [Citation14]. We used the Scale of Chemotherapy-Induced long-term Neurotoxicity (SCIN), which is a brief self-reported scale covering peripheral sensory neuropathy, Raynaud's phenomenon, and ototoxicity [Citation15]. The response categories of all six items had the similar four levels as the questionnaires of HRQOL described above.
The participants also went through a structured telephone interview performed by trained health personnel during the period September 2009 – August 2010. There were predefined questions regarding socio-demographics, comorbidity such as diabetes mellitus, cardiovascular disease, and inflammatory bowel disease (response categories ‘yes’/‘no’), skeleton pain in the lower back or hip (response categories with four levels, from ‘not at all’ to ‘very much’), and lymph oedema (response categories ‘yes’/‘no’).
Reference normative data
Due to the risk of bias in the recruited reference group, a supplementary comparison to existing normative data for the QLQ-C30 from the Norwegian population published 15 years ago [Citation16] was planned. At the start of the analyses more recent normative data from the Netherlands [Citation17] were available. There is a possibility that normative data may change with time, and the Dutch population was considered comparable to the Norwegian population. We therefore decided to perform comparisons with both normative datasets. Reference data were calculated according to the age- and sex-distribution in the present study. As far as we know, there exists no normative data for the QLQ-CR29 or SCIN.
Statistics
Data were analysed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Differences between responders and non-responders were analysed using χ2-tests for categorical variables and Student's t-tests for continuous variables. Analyses of the HRQOL scores including handling of missing values were performed according to the EORTC scoring manual [Citation12]. Mean scores of HRQOL were compared between the groups. The HRQOL symptoms considered relevant in the pre-specified hypothesis were dichotomised as ‘not at all/a little’ (no/mild) versus ‘quite a bit/very much’ (moderate/severe) in analyses of frequency of symptoms. Due to age- and sex-matched volunteers, conditional logistic regression was used to compare the percentage distribution of symptoms between the groups and mean scores of HRQOL. χ2-tests were used in comparisons of subgroups of survivors in analyses of neurotoxicity.
Ethical considerations
All data received were treated in strict confidence. The study was approved by the Regional Committee for Medical Research Ethics of Northern Norway.
Results
Participants
Signed informed consent was obtained from 136 survivors; of these, 128 (64%) returned the questionnaires. The median follow-up time after diagnosis was 66 months (range 25–112). The patient and tumour characteristics of responders and non-responders are described in . The responders were in general younger than the non-responders, and had less comorbidity, but there were no significant differences in TNM staging, radiotherapy dose, or chemotherapy. In all, 21 responders had a stoma (eight due to residual tumour/local recurrence, six due to pre-treatment stoma not reversed, six due to complications, and one unknown).
Table I. Patient-, tumour- and treatment characteristics of the responding and the non-responding survivors.
Signed informed consent was obtained from 278 volunteers, and 269 (22%) returned the questionnaires. A minimum of two volunteers per survivor was obtained, except in the oldest age group of females where the aim was to enrol 50 volunteers, but 45 were obtained. There was no significant difference of self-reported comorbidity between the survivors and the age- and sex-matched volunteers. Current smoking was more common among survivors (30%) than volunteers (11%).
EORTC QLQ-C30
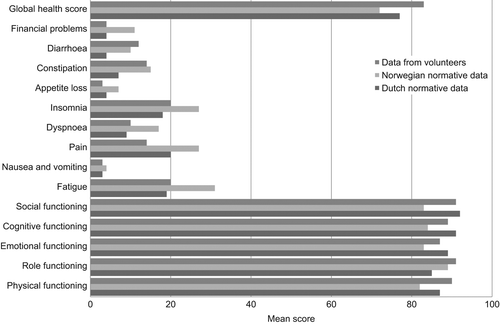
The scores of the survivors were poorer than the scores of the volunteers for all scales and single items (). For the functional scales, the difference was most prominent (≥ 20 points) for role and social functioning (p < 0.001). Survivors reported worse symptom scores than volunteers, and the difference in scores was especially large (≥ 15 points) for fatigue, dyspnoea, insomnia, and diarrhoea (p < 0.001). The global QOL was significantly lower (15 points, p < 0.001) among survivors.
Table II. EORTC QLQ-C30 mean scores of anal cancer survivors compared to volunteers.
To evaluate whether the group of volunteers was a representative sample of the normal population, a comparison between the QLQ-C30 scores of the volunteers and the Norwegian and Dutch normative data corrected for age and sex was performed (). In general all groups followed the same pattern with comparable scores. The present reference group of volunteers was in general more similar to the Dutch normative data than the Norwegian normative data.
EORTC QLQ-CR29
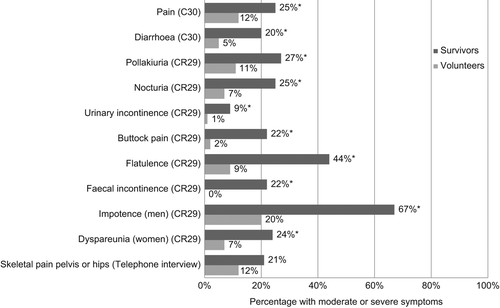
The anal cancer survivors had worse scores than the volunteers for all scales and items of relevance in the colorectal module QLQ-CR29 (). The largest differences in scores (≥ 15 points) were observed for stool frequency, buttock pain, anxiety, flatulence, faecal incontinence, impotence (men), and sexual interest (women) and dyspareunia (p < 0.001).
Table III. EORTC QLQ-CR29 mean scores of anal cancer survivors compared to volunteers.
Symptom burden scored in QLQ-C30, QLQ-CR29 and telephone interview
The percentages of survivors and volunteers reporting moderate to severe symptoms that were considered relevant in the pre-specified hypothesis, are shown in . Moderate to severe symptoms of pain, diarrhoea, urinary frequency and incontinence, buttock pain, flatulence, faecal incontinence, impotence (males), and dyspareunia (females) were more frequent among survivors than volunteers (p < 0.05). Faecal incontinence of any degree (a little to very much) was reported by 57% of the survivors without stoma versus 8% of the volunteers (p < 0.001). Pollakiuria and nocturia were the dominating urinary symptoms, while urinary incontinence and dysuria were less common. Localised pain in the pelvis or hips seemed to be an unspecific symptoms also occurring in the normal population, while pain in general and in particular buttock pain occurred more frequently among survivors compared to volunteers. Analgesics were used daily by 17% of the survivors and 6% of the volunteers (p = 0.005). Lymph oedema was reported by both survivors (10%) and volunteers (4%).
SCIN
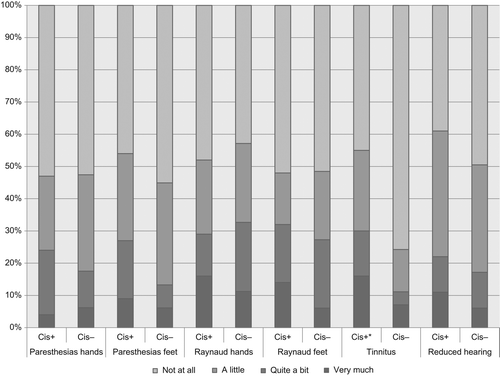
Fifty-six survivors (44%) had received cisplatin as part of their chemoradiotherapy. Of these, most had received a cumulative dose > 200 mg/m2. A comparison between survivors who had received cisplatin to survivors treated without cisplatin revealed more tinnitus in the cisplatin-treated group (p = 0.004) ().
Discussion
Anal cancer survivors who have been treated with chemoradiotherapy have significantly reduced HRQOL in most domains, with impaired function and moderate to severe symptoms, compared with age- and sex-matched volunteers. This reduced HRQOL may be as a result of the disease and/or the treatment. The survivors reported impairment of function, especially social, role, and sexual function as well as increased frequency of fatigue, diarrhoea, buttock pain, flatulence, and faecal incontinence. The global QOL was significantly reduced.
Patients undergoing curative chemoradiotherapy for anal carcinoma have a five-year CSS of 75% [Citation3]; therefore, many patients become long-term survivors. However, the cost for the patient is the long-term sequelae of the disease and treatment toxicity that may have a significant impact on HRQOL. This study shows that social and role function were clearly reduced in long-term anal cancer survivors. Symptoms from the pelvis such as diarrhoea, buttock pain, faecal incontinence, and sexual problems are often perceived as private and embarrassing, and may affect self-confidence and have impact on daily life. The nature and severity of symptoms may negatively affect a person's ability to function and enjoy life [Citation18], and might result in avoidance or isolation [Citation19]. It has been claimed that approximately 50% of survivors after pelvic radiotherapy have their quality of life affected by gastrointestinal symptoms, and 20–40% rate the effects as moderate to severe [Citation20]. Although the exact percentage among anal cancer survivors is not known, there is reason to believe that the situation might be as bad or even worse for this group since the radiation dose is high and the target volume includes or has near proximity to organs such as the anal sphincter and the genitalia. So far, most available data on HRQOL in anal cancer survivors come from small patient samples. Diarrhoea, faecal incontinence and sexual dysfunction are commonly reported [Citation5–9], which was confirmed in the current study. Gastrointestinal dysfunctioning such as diarrhoea, flatulence and faecal incontinence were commonly reported and represent problems that may result in major limitations in daily life. Furthermore, considerably reduced sexual interest and sexual dysfunction were reported among anal cancer survivors. The majority of male anal cancer survivors reported impotence, and a large proportion of women reported dyspareunia. These findings are consistent with results of late effects and HRQOL after radiotherapy for other pelvic malignancies which have revealed significant long-term effects on anorectal and sexual function [Citation21–23].
The total burden of dysfunction and symptoms seems to be high among anal cancer survivors. The mean global health score among survivors was 68, which corresponds fairly well with previous studies on anal cancer [Citation5,Citation6,Citation8]. The age- and sex-matched volunteers reported a mean score of 83, while the normative data from the Norwegian [Citation16] and the Dutch population [Citation17] had mean scores of 72 and 77, respectively. Long-term HRQOL in survivors of other pelvic malignancies have reported slightly better mean global QOL scores within the range of 73–80 [Citation24–26]. HRQOL is complex and characterised as subjective and multi-dimensional. HRQOL assessment does not solely reflect symptom burden, but includes factors not directly related to disease-related or treatment-related effects [Citation18]. Using PROs provide a means of quantifying qualitative information [Citation27]. The questionnaires` limitations must be kept in mind. Adaptation, improved coping, and response shift may contribute to improving or maintaining global QOL [Citation28], and may thus increase the complexity of analyses and interpretation.
There is limited data of neurotoxicity in survivors treated with cisplatin for anal carcinoma. For survivors after testicular cancer, cisplatin may induce neurotoxicity at cumulative doses of 300 mg/m2 [Citation15]. Most cisplatin-treated survivors in the present cohort received a lower cumulative dose, although cisplatin was scheduled as three courses of relatively high doses (60–100 mg/m2). Anal cancer patients are in general older than testicular cancer patients, which might influence the susceptibility to neurotoxicity. Although there was an increased frequency of tinnitus, there seemed to be few problems with neurotoxicity following cisplatin-based chemoradiotherapy for anal carcinoma.
Treatment should be optimised in order to improve outcomes and minimise late effects. Larger tumours are usually more invasive with deeper tumour infiltration and may potentially induce more damage to the sphincter and anal canal. Locally advanced tumours were also treated more extensively, often with larger fields, higher radiation doses, and higher cumulative chemotherapy doses. Intensity-modulated radiotherapy may reduce the radiation dose to organs at risk to some extent [Citation29], particularly the dose to the small intestine, and to some extent the genital organs. The target volume must not be compromised; it encompasses the anal canal, sphincter, rectum and, due to close proximity, parts of the sacral nerves and the internal genital organs as well [Citation30]. In addition, the primary tumour itself may contribute to later dysfunction if the cancer invades the anal sphincter and pelvic floor and the tumour is replaced by fibrotic tissue.
The current study has limitations. The cross-sectional design of the study resulted in variable length of time since diagnosis. This might have implications for the incidence of late effects and curtail the assessment of the development of late effects with time. There is a possible biased selection both among survivors and in particular in the reference group of volunteers. Elderly and survivors with comorbidity were more reluctant to participate. It is unknown whether this has affected the results in any directions, but tumour- and treatment characteristics were fairly similar. Regarding the reference group the concern for bias is greater due to the low response rate, which may lead to a healthier group with a more positive attitude than the actual normal Norwegian population. In a previous, large, Norwegian study the impact of self-selection in population-based surveys was evaluated [Citation31], and characteristics of responders differed only slightly from the target population on variables such as self-rated health, smoking, BMI and mental health. In the current study we were not able to estimate the possible bias in the group of volunteers as we did not have complementary information of the non-responders. In order to minimise this bias, the results from the volunteers were compared with two other sets of normative data.
At the same time, the current study has several strengths. The study population consisted of a large, unselected national cohort of anal cancer survivors. There was information of patient-, tumour- and treatment characteristic of responders and non- responders among the survivors. HRQOL were evaluated with validated, well-known methods, and the outcomes were compared to volunteers not treated for pelvic cancer. To our knowledge this is the largest study of long-term HRQOL in survivors after curative chemoradiotherapy for anal carcinoma.
Long-term follow-up of survivors treated for anal cancer should include evaluation of HRQOL. Although there is increasing knowledge of the prevalence of late effects, there is a need for more research with focus on the severity, consequences, risk factors, prevention, and treatment of late radiation effects. The private and tabooed nature of pelvic symptoms may restrain survivors from mentioning this when unasked. Thankfulness for being alive may also contribute to suppression. Time is often a limiting factor during consultation, representing a priority challenge to health personnel. The recognition of the symptoms and dysfunction may contribute to relief, and help survivors to cope with impaired function and pelvic symptoms. An increased awareness and acceptance of the extent of the problem will stimulate and facilitate multidisciplinary collaboration, which is necessary to improve the care of these cancer survivors.
In conclusion, survivors after chemoradiotherapy for anal cancer have significantly impaired HRQOL in long-term follow-up. Most function and symptom scales were affected. An increased attention and a greater effort to identify and alleviate problems in survivorship of anal cancer are required.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Glynne-Jones R, Northover JM, Cervantes A, group EGW. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010; 21 (Suppl 5):v87–92.
- Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, 3rd, Thomas CR, Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA 2008;299:1914–21.
- Bentzen AG, Guren MG, Wanderas EH, Frykholm G, Tveit KM, Wilsgaard T, et al. Chemoradiotherapy of anal carcinoma: Survival and recurrence in an unselected national cohort. Int J Radiat Oncol Biol Phys 2012;83:e173–80.
- Vistad I, Cvancarova M, Fossa SD, Kristensen GB. Postradiotherapy morbidity in long-term survivors after locally advanced cervical cancer: How well do physicians’ assessments agree with those of their patients? Int J Radiat Oncol Biol Phys 2008;71:1335–42.
- Allal AS, Sprangers MA, Laurencet F, Reymond MA, Kurtz JM. Assessment of long-term quality of life in patients with anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer 1999;80:1588–94.
- Jephcott CR, Paltiel C, Hay J. Quality of life after non- surgical treatment of anal carcinoma: A case control study of long-term survivors. Clin Oncol (R Coll Radiol) 2004;16: 530–5.
- Welzel G, Hagele V, Wenz F, Mai SK. Quality of life outcomes in patients with anal cancer after combined radiochemotherapy. Strahlenther Onkol 2011;187:175–82.
- Provencher S, Oehler C, Lavertu S, Jolicoeur M, Fortin B, Donath D. Quality of life and tumor control after short split-course chemoradiation for anal canal carcinoma. Radiat Oncol 2010;5:41.
- Das P, Cantor SB, Parker CL, Zampieri JB, Baschnagel A, Eng C, et al. Long-term quality of life after radiotherapy for the treatment of anal cancer. Cancer 2010;116:822–9.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality- of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017–26.
- Fayers P, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A, et al. The EORTC QLQ-C30 scoring manual, 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer; 2001.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Cavaletti G, Frigeni B, Lanzani F, Mattavelli L, Susani E, Alberti P, et al. Chemotherapy-Induced Peripheral Neurotoxicity assessment: A critical revision of the currently available tools. Eur J Cancer 2010;46:479–94.
- Oldenburg J, Fossa SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res 2006;15:791–800.
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ = C30 (+ 3). J Clin Oncol 1998;16: 1188–96.
- van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer 2011;47:667–75.
- Burkett VS, Cleeland CS. Symptom burden in cancer survivorship. J Cancer Surviv 2007;1:167–75.
- Norton NJ. The perspective of the patient. Gastroenterology 2004;126:S175–9.
- Andreyev HJ, Wotherspoon A, Denham JW, Hauer-Jensen M.‘Pelvic radiation disease’: New understanding and new solutions for a new disease in the era of cancer survivorship. Scand J Gastroenterol 2011;46:389–97.
- Bruheim K, Tveit KM, Skovlund E, Balteskard L, Carlsen E, Fossa SD, et al. Sexual function in females after radiotherapy for rectal cancer. Acta Oncol 2010;49:826–32.
- Bruheim K, Guren MG, Dahl AA, Skovlund E, Balteskard L, Carlsen E, et al. Sexual function in males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:1012–7.
- Lind H, Waldenstrom AC, Dunberger G, al-Abany M, Alevronta E, Johansson KA, et al. Late symptoms in long-term gynaecological cancer survivors after radiation therapy: A population-based cohort study. Br J Cancer 2011;105: 737–45.
- Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:1005–11.
- Fransson P, Lund JA, Damber JE, Klepp O, Wiklund F, Fossa S, et al. Quality of life in patients with locally advanced prostate cancer given endocrine treatment with or without radiotherapy: 4-year follow-up of SPCG-7/SFUO-3, an open-label, randomised, phase III trial. Lancet Oncol 2009; 10:370–80.
- Ferrandina G, Mantegna G, Petrillo M, Fuoco G, Venditti L, Terzano S, et al. Quality of life and emotional distress in early stage and locally advanced cervical cancer patients: A prospective, longitudinal study. Gynecol Oncol 2012;124:389–94.
- McKenna SP. Measuring patient-reported outcomes: Moving beyond misplaced common sense to hard science. BMC Med 2011;9:86.
- Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: A theoretical model. Soc Sci Med 1999;48:1507–15.
- Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, et al. Dose-painted intensity- modulated radiation therapy for anal cancer: A multi- institutional report of acute toxicity and response to therapy. Int J Radiat Oncol Biol Phys 2012;82:153–8.
- Ng M, Leong T, Chander S, Chu J, Kneebone A, Carroll S, et al. Australasian Gastrointestinal Trials Group (AGITG) contouring atlas and planning guidelines for intensity- modulated radiotherapy in anal cancer. Int J Radiat Oncol Biol Phys 2012;83:1455–62.
- Sogaard AJ, Selmer R, Bjertness E, Thelle D. The Oslo Health Study: The impact of self-selection in a large, population-based survey. Int J Equity Health 2004;3:3.