Abstract
Background. The analysis of regional lymph nodes is particularly relevant in patients with stage II colorectal cancer, in whom the role of adjuvant chemotherapy remains unclear. The aim of this study was to assess the relationship between number of examined lymph nodes and survival in patients with stage IIA (pT3N0M0) colorectal cancer, and to determine the optimal number of lymph nodes that should be examined. Methods. The study group included all the surgically-treated colorectal cancer patients in stage IIA (n = 657) who were identified through the population-based Cancer Registry of the Province of Modena (Northern Italy), during the period 2002–2006. Results. The median number of harvested lymph nodes was 19 (range 1–68). Considering, as a reference point, patients with 12 or less lymph nodes, subjects with n ≥ 20 lymph nodes examined showed, in univariate analysis, a significantly higher cancer specific (p = 0.01) and relapse-free survival (p = 0.003). The results were confirmed by multivariate analysis (Cox model). Conclusion. The result suggests that colorectal cancer patients in stage IIA with n ≥ 20 lymph nodes examined exhibit better survival when compared with subjects in whom fewer lymph nodes were examined. The number of 20 lymph nodes is the essential requirement for an oncologic resection of the large bowel.
Adequate lymph node staging of patients undergoing radical surgery for colorectal neoplasm is essential to establish prognosis and plan further therapies [Citation1–3]. The impact of regional lymph node metastases in stage II colorectal cancer has been widely investigated in studies which, overall, support the primary role of lymph node status in predicting mortality [Citation4–6]. Many studies have suggested that long-term survival increases in parallel with the number of nodes harvested, regardless of the number of positive nodes [Citation3–9]. Although the current American Joint Committee on Cancer guidelines [Citation10,Citation11] recommends the assessment of at least 12 lymph nodes, the minimum number that should be examined is still a controversial point and a wide range of proposals have been reported in the literature [Citation6–12]. The aim of this study was to assess the relationship between number of lymph nodes examined, relapse-free survival (RFS) and cancer-specific survival (CSS) in patients with stage IIA (pT3N0M0) colorectal cancer, and to determine the optimal number of lymph nodes that should be examined.
Material and methods
Study group
Registry
The present population-based study took advantage of the existence of a Cancer Registry in the province of Modena (Northern Italy), which enabled us to select all patients with stage IIA colorectal cancer (ICD-O3 C18-C20 site code), with histological verification, between 2002 and 2006 and who underwent radical surgery. The main sources of information concerning the tumors were the archives of the Pathology Department, and the clinical charts available in the Medical and Surgical divisions of the Health Care District, and collected in the database of the Registry. The quality of the Modena Cancer Registry data relies mainly on a very low rate of Death Certificate Only (DCO) cases, which in the period 2002–2006 was inferior to 0.3% (0.26%). We checked the accuracy of the registration by means of software provided by IARC (IARCcrg Tools, V 2.04) concerning ICD-O3 topography, histology, age, gender and basis of diagnosis. The follow-up completeness was measured according to the method proposed by Wu et al. [Citation13].
Histology
All patients underwent surgical resection of the tumor and regional lymph nodes. The surgical specimens were fixed in 10% formalin and routinely processed for paraffin embedding. Lymph nodes in the specimens were identified by sight and palpation. Routine histopathological examination was carried out using hematoxylin and eosin staining. Tumors were classified as well (G1), moderately (G2) and poorly differentiated adenocarcinoma (G3), following the World Health Organization current guidelines for morphology [Citation14], and staged with the revised TNM system 2011 [Citation15]. According to this system, stage IIA tumors show infiltration through the muscular wall into the pericolic or perirectal fat, but without invasion of the contiguous organs and without reaching the serosa.
Statistical analysis
The principal end-point of the study was RFS, defined as the time between the date of diagnosis and that of relapse or last follow-up for censored data. The secondary end-point was CSS, defined as the time between diagnosis and death from colorectal cancer, or date of last follow-up (for living patients). Other causes of death were censored. The last follow-up was 31 December 2009. RFS and CSS were calculated using the Kaplan-Meier estimators [Citation16]. Univariate and multivariate analyses were carried out with Cox proportional hazard regressions [Citation17], and the proportionality of hazard was checked graphically using the scaled Schoenfeld residuals [Citation18].
The optimum cut-off for the number of lymph nodes was chosen according to the Lausen method applied to the RFS [Citation19]. For every reasonable cut-off point of the number of lymph nodes, the absolute value of standardized log-rank statistic was computed. The value where the standardized log-rank statistic reached its maximum was used as an estimation of the unknown cut-off point. This procedure lead to an optimal separation into two groups: those at low risk of recurrence and those at high risk.
To assess the reliability and accuracy of the cut-off, we performed an internal validation by means of the bootstrap technique. In detail, we performed 250 bootstrapping re-samples where the optimal cut-off in the bootstrap sample was recalculated each time, then validated in out of bootstrap sample.
Moreover, to assess the reproducibility of the cut-off point, we considered, as an external validation, 571 cases with diagnosis of T3N0M0 colorectal cancer recorded in the Modena Cancer Registry between 2000 and 2001 or 2007 and 2009. We carried out the analysis on the CSS alone, because at the time the information on the relapse rates was not available outside of the period 2002–2006.
The χ2, Fisher's exact, and Kruskal-Wallis tests were used to compare the categorical variables; the continuous variables were compared using the coefficient of correlation. All p-values were two-sided. R software version 2.10.0 was used for the calculations pertaining to the cut-off point, while the Stata 10/SE package (StataCorp, College Station, TX, USA) was used for all remaining statistical analysis.
Results
Clinical and histological features
Between January 2002 and December 2006 a total of 2961 patients with colorectal cancer were logged; of these, 666 individuals had a tumor classified as stage IIA. Nine patients with missing data were excluded, and the study was conducted on 657 patients (365 males, 56%; 292 females, 44%; mean age at diagnosis 70.6 years, range 34–95); 535 tumors (82%) were located in the colon and 122 (18%) in the rectum (including the rectosigmoid junction). Clinical and histological data concerning the patients are summarized in . The mean follow-up observation in patients who survived was 62.7 months (median 63; ranging from 27 to 100 months). The completeness of follow-up, taken as the percentage of the maximum possible follow-up at 31 December 2009, was 91% according to Wu et al. 2008 [Citation13].
Table I. Clinical and pathological features of the investigated patients.
Analysis of the lymph nodes
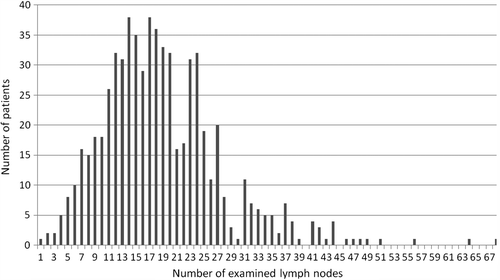
The mean number of harvested lymph nodes was 19 (range 1–68; SD ± 9), with no significant difference between males and females. The distribution of the number of lymph nodes in our study group is showed in . In 121 patients (18.4%), we examined fewer than 12 lymph nodes, and in 536 subjects (81.6%) 12 or more. Right-sided tumors were associated with a significantly higher number of harvested nodes compared to tumors at other sites (mean of 21.5 nodes in right colon, 17.5 in left colon and 18.1 in the rectum; p < 0.001). Increasing age of patients was significantly associated with a lower number of lymph nodes examined (R2 = −0.866; p < 0.001). More in detail, harvesting of 20 or more lymph nodes was carried out in 35% of the patients with age > 70, and in 48% of patients with age < 70 (p = 0.001).
Relapse-free survival
Overall, relapses developed in 87/657 cases (13%); of these, 59/535 (11%) occurred in the colon and 28/122 (23%) in the rectum. The RFS at five years was 86% (95% CI 83–88) and was significantly higher in patients with colon cancer than in individuals with rectal tumors (p = 0.001); the correlation with different clinical and histopathological variables is shown in . Gender, age, grading of tumor, positive margin, tumor size, adjuvant chemotherapy and pre-operative radiotherapy application were not significantly correlated with survival. If we consider the number of lymph nodes examined as a continuous variable, a significant positive correlation was observed between the number of lymph nodes examined and the RFS (p = 0.015).
Table II. Univariate and multivariate analysis of relapse free survival.
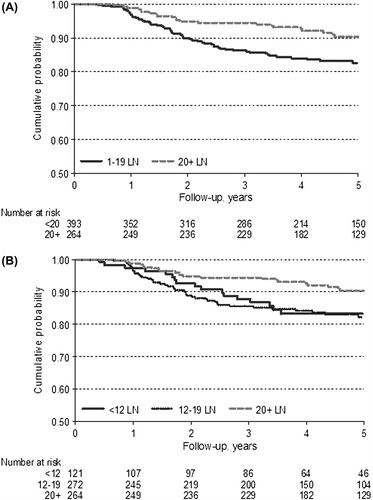
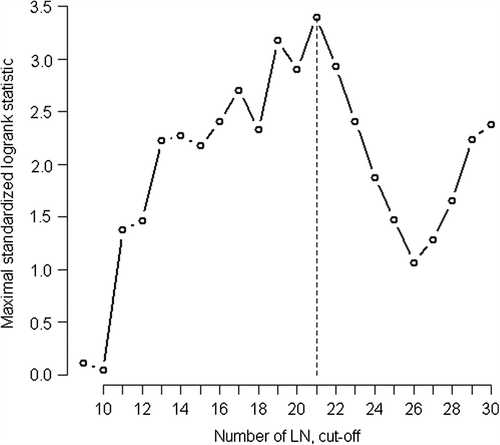
The analyses of our cases revealed that a number of examined lymph nodes of 12 is not sufficient to stratify patients into different categories of relapse risk. As a matter of fact, five-year RFS rate was similar between patients with fewer than 12 lymph nodes examined and patients with over 12 (p = 0.138; CI 0.44–1.12). In order to identify the optimal cut-off number of lymph nodes to be examined for a satisfactory outcome, we adopted the Lausen method [Citation19], which stratifies patients independently of the tumor site (colon and rectum). As reported in , the best adequate cut-off useful to identify two groups with different risk of relapse was 21 lymph nodes [Max Std long-rank = 3.395, p = 0.0092]. Furthermore, using Bootstrapping as a validation method, we suggest that a cut-off between 19 and 21 lymph nodes could be the optimum. Based on these results, we used a number of 20 lymph nodes to carry out further analysis. The stratification of patients with this new cut-off, independently of site, revealed that subjects with 20 or more lymph nodes harvested had a lower risk of relapse, as shown in (HR 0.49; 95% CI 0.30–0.79; p = 0.003). Taking into consideration the guideline number of 12 lymph nodes and our new cut-off of 20, we obtained three patient groups: (< 12 = 121 cases; 12–19 = 272; ≥ 20 = 264 cases). The first two groups showed a similar trend, with overlapping survival curves; patients with more than 20 lymph nodes harvested, however, had a better RFS (). In a multivariate analysis, the value 20 or more was confirmed to be an independent prognostic factor (p = 0.003). Details are shown in .
Figure 2. The optimum cut off of number of harvested lymph nodes. The optimum cut-off of the number of lymph nodes (LN) was chosen according to Lausen method applied to RFS. For every reasonable cut-off point of the number of lymph nodes, the absolute value of standardized log-rank statistic was computed. The value in which the standardized log-rank statistic took their maximum was 21 (Max Std log-rank = 3.395; P = 0.0092). Then 21 lymph nodes represented the optimal cut off that best separates the cases in subgroups at higher and lower risk of recurrence.
Cancer-specific survival
At the last follow-up, 463 patients (70%) were still living. Among the 194 patients who died within five years from diagnosis, 58 died due to disease progression. The CSS at five years was 91%, with 95% CI 88–93. Univariate analysis showed that patients with 20 or more lymph nodes examined had a lower risk of dying of the disease (HR 0.46, 95% CI 0.26–0.82, p = 0.01) and a better CSS (). Moreover, from the analysis of the external validation sample (571 subjects with T3N0M0 colorectal cancer diagnosed between 2000 and 2001 or 2007 and 2009) we could confirm that patients with 20 or more lymph nodes examined showed a lower risk of dying of the disease (p = 0.013) (HR 0.43, CI 95% 0.22–0.85). Among the clinical and pathological variables, increasing age of patients and rectal tumors were associated with a lower CSS at five years (p < 0.001 and p = 0.045). In contrast, we observed better survival in patients who underwent adjuvant chemotherapy (p = 0.011) (). Multivariate analysis confirmed the independent prognostic value of 20 lymph nodes examined as the optimal cut-off, with a 45% reduction in colorectal cancer-related death (HR 0.55, 95% CI 0.30–0.98; p = 0.045). Other independent variables with negative impact on survival were age > 70 years and location within the rectum, but not adjuvant chemotherapy, as shown in .
Figure 4. Cancer-specific survival stratified by number of lymph nodes. CSS curves for all patients, according to number of the lymph nodes examined, stratified as < or ≥ 20.
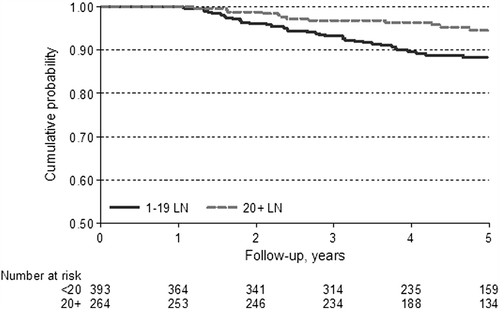
Table III. Univariate and multivariate analysis of cancer specific survival.
Chemotherapy, radiotherapy and surgery
In our study about 30% (189/657) of patients received adjuvant chemotherapy, specifically, 142 subjects with colon cancer (142/535; 27% of total patients with colon cancer) and 47 patients with rectal cancer (47/122; 38% of total patients with rectal cancer). The impact of this treatment suggested an improved CSS but not a benefit in terms of RFS. Patients treated with chemotherapy were younger than untreated individuals. Specifically, among patients aged < 70 years 55% received chemotherapy, while among subjects aged ≥ 70 years only 11% were treated. Stratifying by age, the impact of adjuvant chemotherapy on survival disappears. These results were confirmed by a multivariate analysis in which chemotherapy did not appear as a significant prognostic variable ().
Only 34 patients (of 657, 5%) were treated with pre-operative radiotherapy (RT), while no patients received post-operative radiation. Pre-operative RT did not significantly affect RFS (p = 0.162), CSS (p = 0.349) or pattern of lymph node harvesting (p = 0.859).
Considering only those patients with rectal cancer (122 subjects), pre-operative RT was administered to 27% of subjects (34/122). In this subgroup pre-operative RT did not influence the pattern of lymph nodes harvested stratified by the 20 cut-off (p > 0.50), and did not significantly modify the RFS (p = 0.724) or CSS (p = 0.635).
In our study group, subjects that received a segmental resection of the large bowel (73/657; 11%) had fewer lymph nodes harvested (25% of these patients had 20 or more lymph nodes harvested compared to 42% of the others; p = 0.005) than patients subjected to standard surgery (230 right and 227 left hemicolectomies, five total colectomies, 107 anterior rectal resections and 15 abdominoperineal resections of the rectum). However, the type of surgery did not affect survival (data not reported).
Discussion
The results of the present study can be summarized as follows. First, the total number of lymph nodes harvested is an important prognostic factor for patients with stage IIA (pT3N0M0) colorectal cancer. Second, even if a greater number of lymph nodes examined correlates with better prognosis, a minimum of 12 lymph nodes seems insufficient to stratify patients with different outcome. However, cases with 20 or more nodes examined have a significantly higher RFS and CSS, irrespective of the cancer site.
Lymph node metastasis is one of the most important prognostic factors in surgically-treated colorectal cancer patients; thus, adequate regional (mesenteric) lymph node staging is important for planning treatments, in particular for stage II (Dukes’ B) disease, in which the role of adjuvant chemotherapy remains unclear [Citation1–5].
Although there is a general consensus on pathological examination of at least 12 lymph nodes as the standard at which a colorectal tumor can be considered accurately staged [Citation10], there is a great variation between studies in terms of an ideal minimum number of lymph nodes to be examined in stage II colorectal cancer, with figures ranging from 6 to 24 [Citation1–9,Citation20–27]. There is a general agreement that long-term outcome improves in parallel with the number of nodes examined [Citation20–27]. Among the studies that focused on stage IIa (pT3N0M0), Swanson et al. [Citation3], by evaluating a large cohort of stage IIa colorectal cancer (35 587 cases) demonstrated that their prognosis was dependent on the number of lymph nodes examined, and a minimum of 13 lymph nodes should be examined. Goldstein et al. [Citation20] in a subset of 724 pT3N0 colorectal patients found a significant survival benefit of a higher number of recovered lymph nodes. In particular, patients with 18 or more recovered lymph nodes had a significantly better five-year survival rates than patients with seven or fewer recovered nodes (p = 0.018). The five-year survival rate among patient with more than 18 and 13–17 lymph nodes examined was similar (p = 0.42). However, in this investigation a minimum number of lymph nodes necessary for staging accuracy could not be determined. More recently Torre et al. [Citation25] showed that the number of harvested lymph nodes was the main predictor of survival in stage IIa colorectal cancer patients and that in this sub-group a move beyond staging just 12 nodes or fewer was needed to better stratify relapse risk.
In line with these studies, our results confirm that a greater number of lymph nodes examined are associated with better survival, with a maximum advantage when the number of lymph nodes is ≥ 20. These data are in agreement with the results of Choi et al. [Citation2], who recently reported better survival for patients with 21 or more harvested lymph nodes, when compared with cases with fewer than 21. However, in contrast with this study, which included stage IIA and B, we evaluated only stage IIA cases, in which lymph node staging should represent the most important prognostic factor.
With regard to harvested lymph nodes, a combination of different factors may influence their adequate recovery and examination. These factors include the experience of the surgeons and pathologists as well as tumor- and patient-related factors. The extent of surgical resection is fundamental, because the surgeon should provide an adequate specimen containing the tumor and its mesentery up to the origin of the draining vessels [Citation12,Citation14,Citation25–28]. Although we had no data relative to the actual extent of surgery, based on the type of surgery logged in our population cancer registry, we observed that segmental resection of the large bowel correlated with a lower number of lymph nodes examined but not with survival. Moreover, it is well known that an accurate pathologic dissection of the mesentery contributes to optimal lymph node staging [Citation26–28]. Indeed, it has been reported that nodal metastasis in colorectal cancer is often detected in small lymph nodes (< 5 mm in diameter) [Citation26–28]. A wide number of patient- related factors have been suggested to affect lymph node yield. The immune response to the tumor can produce reactive hyperplasia of the lymph nodes with an increase in their number and dimensions [Citation27–29]. Significant relationships have also been shown between an advanced tumor extension, poor differentiation and increased lymph node retrieval [Citation28–31]. In our study, no statistically significant relation could be demonstrated among these factors and the number of harvested lymph nodes. We reported that older age of patients is significantly associated with lesser nodal retrieval. Age of patients played a large role in the CSS, however, the independent effect on survival of the number of harvested lymph nodes did not disappear. The reason for the associations between age of patients and number of lymph nodes is still unclear but a putative explanation could be the decline of immune competence due to increasing age, and a less extensive bowel resection in older patients [Citation32,Citation33]. Moreover, we observed that right-sided tumors are associated with greater number of harvested lymph nodes than left-sided lesions, and this might be secondary to the removal of longer specimens in right-sided colectomies [Citation32,Citation33].
Although pre-operative RT for rectal cancer can induce a reduction in the number of evaluable lymph nodes [Citation23], we confirm, as observed in previous studies, that the number of examined lymph nodes is independently associated with RFS and CSS in patients who received pre-operative RT for rectal cancer [Citation23].
In conclusion, the results of our investigation suggest that in patients with low-risk stage II (pT3N0M0) colorectal cancer a number of 20 or more lymph nodes is necessary to better identify patients with different prognosis. The etiological explanation of the close correlation between better prognosis and greater number of lymph nodes examined remains unclear; however, our study suggests that examination of 20 or more lymph nodes can be an indirect manifestation either of better tumor-host interaction (and thus of an intrinsically better prognostic factor), or of a more appropriate surgical procedure.
Information on nodal status based on a minimum of 20 lymph nodes and therefore of greater accuracy could be relevant to clinicians when they face a difficult therapeutic decision, as in the case of patients for which the benefit of adjuvant chemotherapy is uncertain. Consequently, surgeons and pathologists should take great care in obtaining and analyzing as many lymph nodes as possible for every patient undergoing surgery for colorectal cancer.
Moreover, the current (7th) edition of the AJCC TNM Cancer Staging Manual [Citation15] defines pN0 as where no regional lymph node metastases are detected, regardless of the number of examined nodes. Our data highlight a relevant prognostic role for the number of lymph nodes in stage IIA colorectal cancer, suggesting that this parameter could be included in the assessment of patient stage.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Stocchi L, Fazio VW, Lavery I, Hammel J. Individual surgeon, pathologist, and other factors affecting lymph node harvest in stage II colon carcinoma. Is a minimum of 12 examined lymph nodes sufficient? Ann Surg Oncol 2011; 18:405–12.
- Choi HK, Law WL, Poon JT. The optimal number of lymph nodes examined in stage II colorectal cancer and its impact of on outcomes. BMC Cancer 2010;10:267.
- Swanson RS, Compton CC, Stewart AK, Bland KL. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003; 10:65–71.
- Sarli L, Bader G, Iusco D, Salvemini C, Mauro DD, Mazzeo A, et al. Number of lymph nodes examined and prognosis of TNM stage II colorectal cancer. Eur J Cancer 2005;41:272–9.
- Vather R, Sammour T, Kahokehr A, Connolly A, Hill A. Lymph node evaluation and long-term survival in Stage II and Stage III colon cancer: A national study. Ann Surg Oncol 2009;16:585–93.
- Chen HH, Chakravarty KD, Wang JY, Changchien CR, Tang R. Pathological examination of 12 regional lymph nodes and long-term survival in stages I-III colon cancer patients: An analysis of 2,056 consecutive patients in two branches of same institution. Int J Colorectal Dis 2010; 25:1333–41.
- Prandi M, Lionetto R, Bini A, Francioni G, Accarpio G, Anfossi A, et al. Prognostic evaluation of stage B colon cancer patients is improved by an adequate lymphadenectomy: Results of a secondary analysis of a large scale adjuvant trial. Ann Surg 2002;235:458–63.
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: Systematic review. J Natl Cancer Inst 2007;99:433–41.
- Peeples C, Shellnut J, Wasvary H, Riggs T, Sacksner J. Predictive factors affecting survival in stage II colorectal cancer: Is lymph node harvesting relevant?Dis Colon Rectum 2010;53:1517–23.
- Specifications of the National Voluntary Consensus Standards for Quality of Cancer Care, Appendix A. National Quality Forum, 2009. Available from: http://www.qualityforum.org/pubblications/2009/05/National_Voluntary_Consensus_Standards_for_Quality_of_Cancer_Care.aspx. [cited 2009 Nov 13].
- Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. National Cancer Institute Expert Panel. Guidelines 2000 for colon and rectal cancer surgery. National Cancer Institute Expert Panel. J Natl Cancer Inst 2001; 93:583–96.
- Scott KWM, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg 1989;76:1165–7.
- Wu Y, Takkenberg JJM, Grunkemeier GL. Measuring follow-up completeness. Ann Thorac Surg 2008;85: 1155–7.
- Hamilton SR, Bosman FT, Boffetta P. Carcinoma of the colon and rectum. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumor of the digestive system, 4th ed. Lyon: IARC Press; 2009. p 133–8.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer staging manual, 7th ed. Springer: New York; 2009.
- Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
- Cox D. Regression models and life tables. J R Stat Soc B 1972;34:187–202.
- Schoenfeld D. Partial residuals for proportional hazard regression model. Biometrika 1982;69:239–41.
- Lausen B, Schumacher M. Maximally selected rank statistics. Biometrics 1992;48:73–85.
- Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: Recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol 2002;26:179–89.
- Tepper JE, O’Connell MJ, Niedzwiecki D, Hollis D, Compton C, Benson AB 3rd, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157–63.
- Cianchi F, Palomba A, Boddi V, Messerini L, Pucciani F, Perigli G, et al. Lymph node recovery from colorectal tumour specimens: Recommendation for a minimum number of lymph nodes to be examined. World J Surg 2002;26: 384–9.
- Tsai CJ, Crane CH, Skibber JM, Rodriguez-Bigas MA, Chang GJ, Feig BW, et al. Number of lymph nodes examined and prognosis among pathologically lymph node-negative patients after preoperative chemoradiation therapy for rectal adenocarcinoma. Cancer 2011;117:3713–22.
- Vather R, Sammour T, Zargar-Shoshtari K, Metcalf P, Connolly A, Hill A. Lymph node examination as a predictor of long term outcome in Dukes B colon cancer. Int J Colorectal Dis 2009;24:283–8.
- La Torre M, Lorenzon L, Pilozzi E, Barucca V, Cavallini M, Ziparo V, et al. Number of harvested lymph nodes is the main prognostic factor in Stage IIa colorectal cancer patients. J Surg Oncol 2012;106:469–74.
- Ko CY, Chang JT, Chaudhry S, Kominski G. Are high- volume surgeons and hospitals the most important predictors of in-hospital outcome for colon cancer resection?Surgery 2002;132: 268–73.
- Wright FC, Law CH, Last L, Khalifa M, Arnaout A, Naseer Z, et al. Lymph node retrieval and assessment in stage II colorectal cancer: A population-based study. Ann Surg Oncol 2003;10:903–9.
- Evans MD, Barton K, Rees A, Stamatakis JD, Karandikar SS. The impact of surgeon and pathologist on lymph node retrieval in colorectal cancer and its impact on survival for patients with Dukes’ stage B disease. Colorectal Dis 2008;10:157–64.
- Ratto C, Sofo L, Ippoliti M, Merico M, Bossola M, Vecchio FM, et al. Accurate lymph-node detection in colorectal specimens resected for cancer is of prognostic significance. Dis Colon Rectum 1999;42:143–54.
- Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: A population-based study. J Natl Cancer Inst 2005;97:219–25.
- Johnson PM, Malatjalian D, Porter GA. Adequacy for nodal harvest in colorectal cancer: A consecutive cohort study. J Gastrointest Surg 2002;6:883–8.
- Carloss H, Huang B, Cohen A, Carloss E, Wyatt S, Tucker T. The impact of number of lymph nodes removed on five-year survival in stage II colon and rectal cancer. J Ky Med Assoc 2004;102:345–7.
- Bui L, Rempel E, Reeson D, Simunovic M. Lymph node counts, rates of positive lymph nodes, and patient survival for colon cancer surgery in Ontario, Canada: A population-based study. J Surg Oncol 2006;93:439–45.