Abstract
Background. Glioma recurrence frequently occurs close to the marginal area of the surgical cavity as a result of residual infiltrating glioma cells. Fluorescence-guided surgery with 5-aminolevulinic acid (ALA) for resection of gliomas has been used as an effective therapeutic approach to discriminate malignant tissue from brain tissue and to facilitate patient prognosis. ALA-based photodynamic therapy is an effective adjuvant treatment modality for gliomas. However, insufficient protoporphyrin IX (PpIX) accumulation may limit the applicability of fluorescence-guided resection and photodynamic therapy in the marginal areas of gliomas. Methods. To be able to understand how to overcome these issues, human glioma cells and normal astrocytes were used as the model system. Glioma cells and astrocytes were preconditioned with calcitriol for 48 hours and then incubated with ALA. Changes in ALA-induced PpIX fluorescence and cell survival after light exposure were assessed. Furthermore, expression of porphyrin synthetic enzymes in pretreated glioma cells was analyzed. Results. Calcitriol can be administered prior to ALA as a non-toxic preconditioning regimen to significantly enhance ALA-induced PpIX levels and fluorescence. This increase in PpIX level was detected preferentially in glioma versus normal cells. Also, calcitriol pretreated glioma cells exhibited increased cell death following ALA-based photodynamic therapy. Furthermore, mechanistic studies documented that expression of the porphyrin synthesis enzymes coproporphyrinogen oxidase was increased by calcitriol at the mRNA level. Conclusion. We demonstrated for the first time a simple, non-toxic and highly effective preconditioning regimen to selectively enhance PpIX fluorescence and the response of ALA-PDT in glioma cells. This finding suggests that the combined treatment of glioma cells with calcitriol plus ALA may provide an effective and selective therapeutic modality to enhance ALA-induced PpIX fluorescent quality for improving discrimination of tumor tissue and PDT efficacy.
Malignant gliomas exhibiting local invasiveness show poor prognosis despite treatment with a combination of surgery, chemotherapy, and ionizing radiation [Citation1]. It has been previously shown that increased survival is related to the extent of surgical resection [Citation2]. However, it is difficult to introparetively distinguish tumor tissue from normal brain due to the infiltrative nature of the tumor, and recurrence of glioma occurs within − 2 cm of the resected margin [Citation3]. Therefore, methods that distinguish malignant tissue from surrounding normal tissue during surgical resection are necessary and valuable.
Fluorescence-guided resection (FGR) with 5-aminolevulinic acid (ALA) enables intraoperative discrimination of viable tumor margins and improves the extent of tumor resection [Citation4]. In addition, ALA-based photodynamic therapy (PDT) has been proven to be an effective adjuvant treatment option for various cancers, including malignant glioma [Citation5]. Combined with photodynamic therapy as an adjunct to surgery, the complete destruction of tumor cells may be achieved.
ALA is a precursor of porphyrins that is converted into the photosensitizer, protoporphyrinIX (PpIX), through the heme biosynthetic pathway [Citation6]. PpIX can lead to photosensitization upon exposure to light, thus forming an ALA-based FGR and PDT [Citation7].
However, even if these methods show great promise, the marginal areas show vague fluorescence because the density of the glioma cells is low and heterogeneous, resulting in insufficient PpIX accumulation [Citation8].Vague fluorescence signals reduce sensitivity and specificity for the detection of malignant tissue [Citation9]. However, the application of ALA-PDT have yet to be widely established in clinical treatment for glioma. This may partly be due to insufficient PpIX levels in the marginal areas containing infiltrating glioma cells in preclinical settings [Citation10]. Thus methods that can increase the accumulation of ALA-induced PpIX are still needed. Our goal then has been to develop new methods that can enhance PpIX accumulation in glioma cells to improve the accuracy of vague fluorescence discrimination and 5-ALA-PDT efficacy.
Previous studies have shown that the intensity of PpIX fluorescence correlates with the expression of coproporphyrinogen oxidase (CPO), one of the porphyrin-synthetic enzymes. Methotrexate was shown to increase ALA-induced PpIX accumulation through a mechanism involving enhanced expression of CPO [Citation11].
In this paper, another molecule, 1, 25(OH)2 vitamin D3, can inhibit cell proliferation in glioma cells [Citation12]. Interestingly, in addition to its potent antitumor properties, it can also increase ALA-induced PpIX accumulation and enhance the effect of PDT in LNCaP prostate cancer cells [Citation13]. Unfortunately, in other tumor cells, this phenomenon has not been observed consistently. For example, when skin keratinocytes were pretreated with vitamin D3, increased accumulation of ALA-induced PpIX was not observed in cell culture [Citation14].
Therefore, our aim in the current study was to investigate whether 1, 25(OH)2 vitamin D3 could selectively enhance the molecular imaging quality of ALA-induced PpIX and potentiate the effect of ALA-PDT in glioma cell lines.
Material and methods
Source of reagents
5-ALA and 1,25(OH)2 vitamin D3 (Calcitriol) were obtained from Sigma-Aldrich (St. Louis, MO, USA). ALA was dissolved in phosphate buffered saline, sterilized by filtration through 0.2 mm pore size filters, and then stored at − 20°C.
Chloroform and methanol were purchased from Merck (Darmstadt, Germany). Dimethylsulfoxide (DMSO) and 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
The human glioma cell lines U87 and T98 were maintained in Dulbecco's modified Eagle's medium (DMEM; Hyclon Logan, UT, USA) supplemented with 10% fetal bovine serum, 100 u/ml penicillin, and 100 u/ml streptomycin and incubated at 37°C in a humidified atmosphere with 5% CO2. Wistar rat cortical astrocytes were cultured in wistar rat cortical astrocytes growth medium (Cyagen, USA) at 37°C in a humidified CO2 incubator. To examine cell viability, glioma cells were mixed with the same volume of 0.4% trypan blue solution, 48 hours after addition of various concentrations of calcitriol. These cells were immediately examined to determine whether they excluded the dye under light microscopic observation.
Quantitative real-time-PCR (QRT-PCR)
Total RNA of glioma cells was extracted with TRIzol (invitrogen) reagent according to the manufacturer's instructions. First-strand cDNA was synthesized by using the high-capacity cDNA reverse transcription kit (Takara) with random primers.
To examine changes in the level of mRNA for PBGD, UROS, CPOX and FECH, QRT–PCR was performed on a LightCycler 480 (Roche, Basel Switzerland) using SYBR Green PCR Master Mix (Toyobo, Osaka, Japan). The primer pairs of these genes are listed in . Parallel reactions were performed using primers to GAPDH as an internal control.
Table I. QRT-PCR primers to quantitatively measure the mRNA levels of the PBGD, UROS, CPOX, and FECH genes.
Fluorescence molecular images of protoporphyrin IX
Glioma cells were plated in a single layer on microscope coverslips, and 24 hours later, the culture medium was replaced with either with medium alone or with medium containing calcitriol. The cells were further incubated for 48 hours. The serum-free DMEM containing ALA solution (final concentration: 0.3 mM) was added to the wells for a final 16 hours of incubation. PpIX fluorescence in the living glioma cells was analyzed by fluorescence microscopy. Intracellular PpIX fluorescence was excited at 488 nm and molecular images were captured in the red channel through a 590 nm long-pass filter.
Fluorescence intensity of PpIX in living cells
Fluorescence intensity of living cells was analyzed by flow cytometry (FACS Calibur; Becton Dickinson, San Jose, CA, USA). Cells were plated and treated under conditions identical to those used for quantifying PpIX. The excitation wavelength used was 488 nm, and the red fluorescence emission of 10 000 cells was recorded through a 670 nm long-pass filter.
Fluorescence intensity of PpIX in cell lysates
Cell extraction procedure
Briefly, glioma cells were plated in single layers on wells, and 24 hours later the medium was replaced with either medium alone or with medium containing calcitriol. The cells were further incubated for 48 hours. The serum-free DMEM containing ALA solution was added to the wells for a final 16 hours of incubation. Treated cells were collected, and the protocol used for cell lysate extraction was adopted from Smits et al. [Citation15] and as described previously [Citation16].
Spectrofluorometric analysis
The PpIX content of cell extracts was assessed by fluorescence spectrophotometry (LS-55, PerkinElmer, Waltham, MA, USA) as described previously [Citation15]. Excitation wavelength was set to 410 nm (slit 10 nm), and emission was recorded at 633 nm (slit 10 nm). An emission spectrum was recorded at the range of 600–750 nm. This fluorescence analysis was performed in the dark to minimize PpIX loss due to photobleaching.
Photodynamic treatment
Glioma cells were seeded into 96-well plates at a density of 1 × 103 per well and incubated with calcitriol for 48 hours. Cells were then washed with PBS and serum-free DMEM containing ALA was added for further incubation with cells for 16 hours. Glioma cells were then irradiated at a power density of 30 mW/cm2 (XD-635AB; Xingda, Guilin, China). Immediately following irradiation, the medium was replaced with DMEM supplemented with 10% FCS, and cells were incubated for 24 hours.
Cell viability assay
Cell viability was examined by the MTT assay. Glioma cells were incubated with medium containing MTT for 4 hours in the dark at 37°C. One hundred microliters DMSO was added into each well after removing the medium and incubated for 20 minutes. UV absorption was measured at 492 nm using a 96-well plate reader. Three independent experiments were conducted.
Cell apoptosis analysis
The apoptosis ratio was analyzed using Annexin V FITC Apoptosis Detection Kit (Beijing, China) according to the manufacturer’s instructions. Apoptotic cells were analyzed and quantified using FACScan flow cytometry (Becton Dickinson, USA). Tests were repeated in triplicate.
Statistical analysis
Statistical analyses were performed using the SPSS13.0 software (SPSS Inc., Chicago, IL, USA). Student's t-test was applied when appropriate. A probability of < 0.05 was considered statistically significant. All values are expressed as mean ± SEM from three different experiments.
Results
Calcitriol at non-cytotoxic concentrations does not increase PpIX levels in glioma cells without ALA stimulation
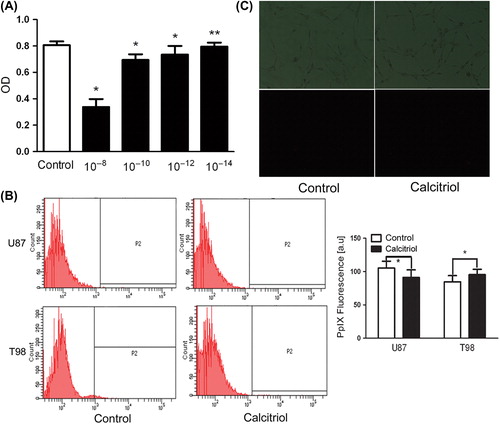
The sensitivity of U87 and T98 glioma cells to caltriciol was first investigated by trypan blue dye exclusion. shows that the cytotoxic effect of a 48 hour-treatment with calcitriol is dose-dependent, and calcitriol at 10214 M was non-cytotoxic. Therefore, 10214 M calcitriol was considered optimal for use with glioma cells. To determine whether a lower calcitriol concentration affects accumulation of PpIX in glioma cells treated without ALA, glioma cells were exposed to calcitriol for 48 hours and PpIX level was assessed by flow cytometry () and fluorescence microscopy (). A low concentration of calcitriol did not induce accumulation of PpIX. Therefore, calcitriol at non-cytotoxic concentrations does not enhance PpIX level in glioma cells treated without ALA.
Figure 1. Effects of calcitriol on viability and intracellular PpIX fluorescence of glioma cells. Glioma cells were pretreated during 48 hours with vehicle alone (control) or with calcitriol at the following concentrations: 1028, 10210, 10212, 10214. Cell viability was estimated with an MTT assay (A). Glioma cells were incubated in culture medium containing calcitriol (10214) for 48 hours. Fluorescence of living cells was analyzed by flow cytometry (B) and fluorescence microscopy (C). Upper panels: phase-contrast images. Lower panels: PpIX-fluorescent images. Data are presented as mean ± SEM for three separate experiments performed in duplicate. Asterisks indicate statistical significance (*p > 0.05, **p < 0.05).
Calcitriol treatment of glioma cells selectively increases ALA-induced PpIX levels
Next, we investigated whether calcitriol pre- treatment causes abundant accumulation of ALA-based PpIX. Glioma cells were preincubated with 10214 M calcitriol for 48 hours, and then the cells were exposed to 0.3 mM ALA. Results from flow cytometry analysis showed that calcitriol-pretreated cells had a higher intracellular concentration of ALA- induced PpIX than control cells (; p < 0.05). We further examined the intracellular level of ALA-induced PpIX by spectrofluorometry, and the result was consistent with that from the flow-cytometric analysis (). One of the important factors determining the success of cancer therapy is the ability to selectively kill tumor cancer cells while sparing normal cells. Therefore, we investigated whether pretreatment of normal astrocytes with calcitriol affects the intracellular level of ALA-induced PpIX. No effect on the accumulation of ALA-induced PpIX was observed in normal astrocytes (). This suggests that calcitriol selectively increased the intracellular PpIX level in glioma cells after ALA exposure.
Figure 2. Effect of calcitriol upon ALA-mediated PpIX accumulation. Glioma cells (A,B) and rat normal astrocytes (C) were incubated for 48 hours in the absence or presence of calcitriol, then ALA (0.3 mM) was added for 16 hours. Intracellular ALA-induced PpIX fluorescence was analyzed by flow cytometry (A, C) and fluorescence spectrophotometry (B). Data are presented as mean ± SEM for three separate experiments performed in duplicate. Asterisks indicate statistical significance (*p > 0.05, **p < 0.05).
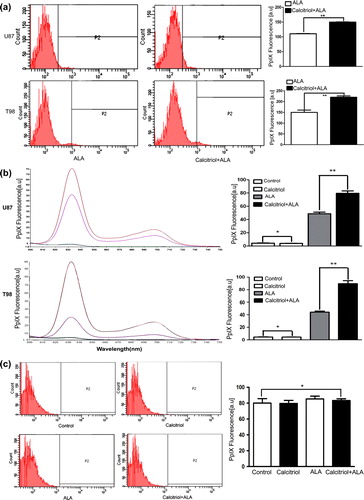
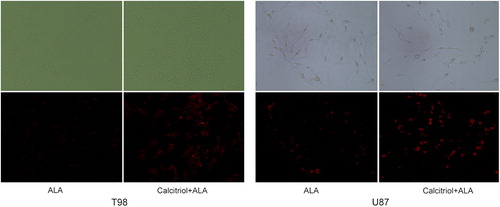
Figure 3. PpIX molecular images of U87 and T98 glioma cells pretreated with calcitriol. Glioma cells were pretreated with vehicle or with calcitriol for 48 hours, and ALA was added to the wells and the cells were incubated for an additional 16 hours. Protoporphyrin IX-specific fluorescence was analyzed by fluorescence microscopy. Upper panels: phase-contrast images. Lower panels: PpIX-fluorescent images.
Calcitriol enhances ALA-induced PpIX molecular imaging quality
We then investigated whether calcitriol could enhance visualization of PpIX in glioma cells. shows the fluorescence images obtained from cells treated with ALA alone and cells treated with both calcitriol and ALA. The molecular imaging quality of PpIX fluorescence in calcitriol-pretreated cells was higher than in control. A high-contrast strong red fluorescence was diffusely localized to the cytoplasm of glioma cells. This increase in fluorescence is consistent with the measured level of PpIX. These data show that we can obtain a high-contrast strong red fluorescence of PpIX in glioma cells pretreated with calcitriol.
Calcitriol pretreatment increases ALA-induced phototoxicity
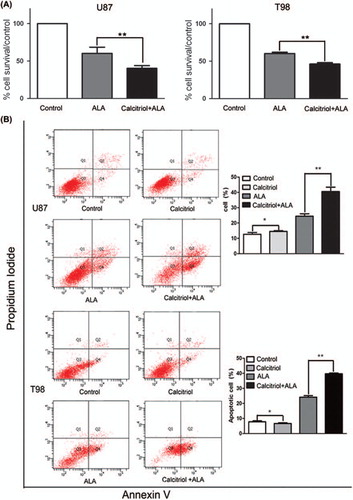
To determine whether calcitriol-pretreated cells with higher photosensitizer levels can increase phototoxicity after exposure to visible light, we subjected glioma cells to calcitriol pretreatment for 48 hours, incubation with 0.3 mM ALA for 16 hours, and then exposure to light. We assessed phototoxicity using two different assays, MTT assay and flow-cytometric analysis. MTT assay results show that calcitriol pretreatment significantly decreased cell viability as compared with controls (; p < 0.05). Inhibition of growth in calcitriol-pretreated U87 or T98 cells was 20% and 15%, respectively. Results from flow-cytometric analysis show that calcitriol significantly increased the number of annexin V- positive apoptotic cells induced by ALA-PDT as compared with control cells (; p < 0.05). Our findings therefore suggest that pretreatment with calcitriol may represent a potential approach to potentiating the effect of ALA-PDT on glioma cells.
Figure 4. Pretreatment of glioma cells with calcitriol enhances cytotoxicity after the addition of ALA and light. U87 and T98 glioma cells were preconditioned for 48 hours with calcitriol or medium alone, then incubated with ALA (0.3 mM) for 16 hours and irradiated with light. (A) Survival was measured by MTT dye assay. (B) Cell apoptosis was analyzed by flow cytometry. Data are presented as mean ± SEM for three separate experiments performed in duplicate. Asterisks indicate statistical significance. (*p > 0.05, **p < 0.05).
Calcitriol enhances the accumulation of ALA-induced PpIX via upregulation of CPOX
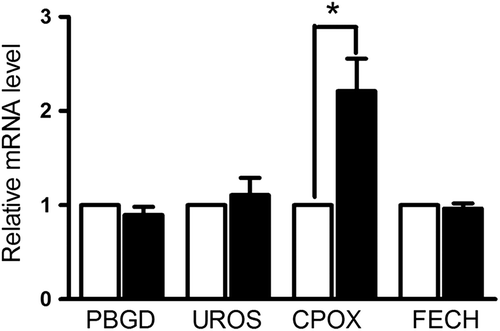
To investigate the mechanism of enhanced intracellular PpIX level following calcitriol pretreatment, we examined the expression levels of rate-limiting enzymes upstream and downstream of PpIX in the heme synthesis pathway. T98 glioma cells were pretreated with calcitriol or with medium alone, and changes in the levels of the porphyrin biosynthetic enzymes (PBGD, UROS, CPOX and FECH) were analyzed by real time-PCR. Only the expression level of copropopyrinogen oxidase was significantly altered after calcitriol pre-treatment (). The mRNA expression level of copropopyrinogen oxidase was increased by approximately one-fold, while PBGD, UROS and FECH showed no changes in the mRNA level. We conclude that calcitriol treatment can significantly increase CPOX expression at the mRNA level. Therefore, calcitriol enhances ALA-induced PpIX level and fluorescence in glioma cells via upregulation of CPOX expression.
Figure 5. mRNA expression of key enzymes in the porphryrin synthetic pathway, with or without calcitriol pretreatment. Glioma cells were pretreated with vehicle or with calcitriol for 48 hours, and mRNA expression of PBGD, UROS, CPOX and FECH were measured by real-time PCR. Data are presented as mean ± SEM for three separate experiments performed in duplicate. Asterisks indicate statistical significance (*p < 0.05).
Discussion
Despite advances in surgical procedures, radiotherapy and chemotherapy, the prognosis of malignant glioma still remains poor due to its invasive character [Citation1]. It has been previously demonstrated that the survival benefit of glioma patients is associated with the extent of resection [Citation2]. However, it is often difficult to distinguish tumor from normal brain tissue during surgical resection using existing methods. This is further complicated by the fact that gliomas often recur in or close to the wall of the resection cavity. Fluorescence-guided surgery with ALA was shown to increase the extent of resection from 36% to 65% [Citation4]. In addition, the application of ALA-PDT has been established in the clinical treatment for glioma. Unfortunately, these techniques are limited in their applicability because ALA-induced PpIX accumulation is insufficient within invasive marginal areas. Therefore, a promising approach to optimize current protocols is to increase accumulation of PpIX within tumor cells.
In the current study, our data indicate that a new combined approach is indeed feasible. Glioma cells pretreated with calcitriol potentiated ALA-induced PpIX accumulation and increased PpIX fluorescence in glioma cells. In addition, we also demonstrated that caltriciol itself could not enhance the levels of intracellular PpIX without ALA. No specific red fluorescence was captured in glioma cells pretreated with calcitriol alone. This suggests that calcitriol has no potential for enhancing physiological PpIX accumulation in glioma cells under normal conditions without ALA. One of the critical factors determining the success of using FGR is its ability to selectively increase PpIX fluorescence in glioma cells while sparing normal cells. Next, we investigated whether calcitriol pretreatment in normal astrocytes enhances PpIX accumulation. Pretreatment of normal astrocytes did not induce any significant increase in ALA-induced PpIX. This also suggests that the use of calcitriol may provide an approach to selectively increase intraoperative ALA-induced fluorescence and improve PpIX fluorescence discrimination accuracy. Depending on the intensity of the fluorescence in tumor cells, neurosurgeons using a surgical microscope with an in-built fluorescence module can potentially obtain detailed diagnostic information and would potentially achieve the best fluorescence-guided discrimination of tumor tissues. Therefore, the approach could more precisely define intraoperative tumor borders and thus achieve maximal cytoreductive surgery without neurologic deficits for patients with glioma in non-functional cortical areas. To enable safe resection of malignant gliomas located adjacent to eloquent areas, some operative support techniques, e.g. navigation systems and functional magnetic resonance imaging (MRI) were used to provide information about the functional locations in the brain, and fluorescence imaging technique was used to distinguish tumors from normal brain tissue. The combination of fluorescence-guided resection and intraoperative evaluation by functional MRI significantly increased the extent of glioma resection without incurring postoperative neurological deficits [Citation17].
Furthermore, we examined the effect of calcitriol on ALA-PDT efficacy in glioma cells. ALA-PDT requires three elements: light, a photosensitizer and oxygen [Citation18,Citation19]. In our study, calcitriol significantly increased cell death after treatment with ALA-PDT with all parameters unchanged except for the photosensitizer (PpIX) concentration. This suggests that calcitriol potentiated the impact of ALA-PDT primarily by enhancing the accumulation of ALA- induced PpIX rather than altering others parameters. The success of PDT depends on the extent by which it selectively kills malignant tumor cells while sparing normal cells. Here, we found that pretreatment of normal astrocytes with calcitriol did not induce any significant increase in ALA-induced PpIX. Therefore, pretreatment with calcitriol could not increase cell death after treatment with ALA-PDT (data not shown). Although the ability of calcitriol to elevate PpIX levels in LNCaP prostate cancer cells was described previously [Citation13], our new data are significant and distinct because we demonstrated for the first time a tumor-selective PpIX-inducing effect of calcitriol in glioma cells.
To examine the mechanism underlying elevation of PpIX levels, we examined the changes in the expression levels of the porphyrin-synthetic enzymes. Unfortunately, we did not detect any changes in the transcript levels of several enzymes, including PBGD, UROS and FECH. However, CPOX mRNA level was significantly higher in calcitriol-pretreated cells than in control. CPOX is located at the mitochondrial outer membrane and plays a role in catalyzing coproporphyrinogen III to produce protoporphyrinogen [Citation20,Citation21]. Previous studies have demonstrated that calcitriol or MTX increases the expression of CPOX, which results in a significant increase in the intracellular accumulation of ALA-induced PpIX in epithelial cancer cells and prostate cancer cells. As a consequence, photodynamic therapy effect is enhanced in preconditioned cells. A similar result was also observed in calcitriol-pretreated glioma cells. In addition, it has previously been suggested that the high mRNA level of CPOX significantly correlated with ALA-induced fluorescence in malignant gliomas [Citation22]. We conclude that the induction of CPOX gene expression plays a role in the ALA-based fluorescence of tumor cells and the effect of photodynamic therapy.
In summary, we have demonstrated for the first time that calcitriol can enhance the quality of ALA fluorescence imaging for optimizing subjective fluorescence discrimination and can improve the efficacy of ALA-PDT by increasing intrinsic PpIX levels. Furthermore, we demonstrated that calcitriol not only can increase the level of ALA-induced PpIX, but it can also confer a tumor-specific increase in photosensitizer levels by elevating CPOX mRNA expression. Future studies are needed to investigate whether calcitriol can be effectively combined with ALA-induced fluorescence-guided surgery and/or ALA-PDT in vivo and in the clinical setting.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (30973078 S.Z. 81272788 S.Z. 81101616 Y.Z. 81201974 L.T. 81302176 X.C.) and Foundation of the first affiliated hospital of Harbin Medical University (2013B05 X.C.). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wen PY, Kesari S. Malignant gliomas in adults. New Engl J Med 2008;359:492–507.
- Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008;62: 753–64.
- Aydin H, Sillenberg I, von Lieven H. Patterns of failure following CT-based 3-D irradiation for malignant glioma. Strahlenther Onkol 2001;177:424–31.
- Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401.
- Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, Tonn J, et al. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol 2007;26:157–64.
- Inoue H, Kajimoto Y, Shibata MA, Miyoshi N, Ogawa N, Miyatake S, et al. Massive apoptotic cell death of human glioma cells via a mitochondrial pathway following 5-aminolevulinic acid-mediated photodynamic therapy. J Neuro-oncol 2007;83:223–31.
- Eljamel MS. Brain photodiagnosis (PD), fluorescence guided resection (FGR) and photodynamic therapy (PDT): Past, present and future. Photodiagn Photodynam Ther 2008;5:29–35.
- Utsuki S, Oka H, Sato S, Suzuki S, Shimizu S, Tanaka S, et al. Possibility of using laser spectroscopy for the intraoperative detection of nonfluorescing brain tumors and the boundaries of brain tumor infiltrates. Technical note. J Neurosurg 2006;104:618–20.
- Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H. 5-aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: A one-year experience at a single institution. Swiss Med Wkly 2008;138:180–5.
- Madsen SJ, Angell-Petersen E, Spetalen S, Carper SW, Ziegler SA, Hirschberg H. Photodynamic therapy of newly implanted glioma cells in the rat brain. Laser Surg Med 2006;38:540–8.
- Anand S, Honari G, Hasan T, Elson P, Maytin EV. Low-dose methotrexate enhances aminolevulinate-based photodynamic therapy in skin carcinoma cells in vitro and in vivo. Clin Cancer Res 2009;15:3333–43.
- Baudet C, Chevalier G, Naveilhan P, Binderup L, Brachet P, Wion D. Cytotoxic effects of 1 alpha,25-dihydroxyvitamin D3 and synthetic vitamin D3 analogues on a glioma cell line. Cancer Lett 1996;100:3–10.
- Ortel B, Sharlin D, O’Donnell D, Sinha AK, Maytin EV, Hasan T. Differentiation enhances aminolevulinic acid- dependent photodynamic treatment of LNCaP prostate cancer cells. Br J Cancer 2002;87:1321–7.
- Sato N, Moore BW, Keevey S, Drazba JA, Hasan T, Maytin EV. Vitamin D enhances ALA-induced protoporphyrin IX production and photodynamic cell death in 3-D organotypic cultures of keratinocytes. J Investig Dermatol 2007;127:925–34.
- Smits T, van Laarhoven AI, Staassen A, van de Kerkhof PC, van Erp PE, Gerritsen MJ. Induction of protoporphyrin IX by aminolaevulinic acid in actinic keratosis, psoriasis and normal skin: Preferential porphyrin enrichment in differentiated cells. Br J Dermatol 2009;160:849–57.
- Zhao SG, Chen XF, Wang LG, Yang G, Han DY, Teng L, et al. Increased expression of ABCB6 enhances 17 protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol 2013;20:4379–88.
- Eyupoglu IY, Hore N, Savaskan NE, Grummich P, Roessler K, Buchfelder M, et al. Improving the extent of malignant glioma resection by dual intraoperative visualization approach. PloS One 2012;7:e44885.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nature Rev Cancer 2003;3:380–7.
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889–905.
- Heinemann IU, Jahn M, Jahn D. The biochemistry of heme biosynthesis. Arch Biochem Biophys 2008;474:238–51.
- Hamza I. Intracellular trafficking of porphyrins. ACS Chem Biol 2006;1:627–9.
- Takahashi K, Ikeda N, Nonoguchi N, Kajimoto Y, Miyatake S, Hagiya Y, et al. Enhanced expression of coproporphyrinogen oxidase in malignant brain tumors: CPOX expression and 5-ALA-induced fluorescence. Neuro-oncology 2011;13:1234–43.
