Abstract
Background. To characterize the stage-specific prognostic relevance of preoperative systemic inflammatory response, defined by C-reactive protein (CRP), in colon cancer (CC) patients. Material and methods. Data from CC patients operated on from 1998 to 2007 at three hospitals from three different Nordic countries were collected retrospectively from national registries, local databases and/or patient records. Patients with emergency surgery, infection or auto-immune disease were excluded. Associations between clinical or histopathological variables and CRP were assessed. Patients were followed from the date of surgery to death or end of follow-up. Disease-specific survival (DSS) was the main endpoint. Results. In total, 525 patients with age and stage distributions which were representative for CC patients were included. None of the patients was lost to follow-up. Age, TNM Stage, WHO differentiation grade and right-sided tumor location significantly associated with elevated CRP values, in contrast to postoperative morbidity, which did not. CRP levels were found to be a strong prognostic factor for DSS in CC. The risk of death due to CC was augmented with increasing levels of CRP in every stage of operated CC. Both short- and long-term DSS were impaired. The sub-hazard ratios for CRP-levels above 60 mg/L were 7.37 (CI 2.65–20.5) for stage I+ II, compared to 3.29 (CI 1.30–8.29) for stage III and 2.24 (CI 1.16–4.35) for stage IV. Conclusion. Increase of CRP concentrations correlate with clinically relevant poorer disease-specific survival in each stage of CC.
Colon cancer (CC) is the third most common cause of cancer death in Western Europe and North America [Citation1].
Interactions between the tumor and the host are increasingly recognized as important determinants of the clinical course. The status of the host's immune system, in particular, has been shown to be of both prognostic and therapeutic relevance in CC and other forms of cancer [Citation2]. Both beneficial immune responses and detrimental pro-inflammatory immune responses have been reported [Citation3].
A pro-inflammatory immune response to the tumor might provoke a systemic inflammatory response (SIR) in the host [Citation3]. A SIR can be detected by measuring preoperative increases in circulating acute phase protein levels such as C-reactive protein (CRP) [Citation3].
Some studies have not found an elevated CRP level to be an independent negative predictor of survival [Citation4], while others report a prognostic significance [Citation5,Citation6] in colorectal cancer and suggest this indicates the presence of a detrimental SIR. The latter reports are mostly derived from single-center studies, and analyze colon and rectal cancer together, despite the fact that rectal cancer has been reported to be associated with lower CRP values [Citation6].
To the best of our knowledge, only one large prospective multicenter study has investigated the prognostic value of CRP [Citation6]. That study was primarily designed as a randomized trial assessing the effect of a histamine-2 receptor antagonist on long-term survival in CRC patients therefore one cannot exclude the possibility that ranitidine might have affected inflammation and CRP levels.
Additionally, data have not been sub-analyzed for long-term survival to determine whether the SIR phenomenon, mirrored by CRP, is a marker of impaired tumor resistance or a marker of more aggressive tumor behavior. It is important, therefore, to investigate any possible prognostic association between preoperative CRP levels and postoperative morbidity and early death from CC. In particular, a prognostic range of CRP values and its relation to tumor stage would be of importance for clinical decision guidance [Citation6].
The aim of this study was to characterize the stage-specific prognostic relevance of preoperative SIR in CC patients. Thus, the association of CRP values with the short-term outcome and long-term DSS of CC patients from three institutions in Sweden, Finland and Norway were analyzed. We hypothesized that a detrimental immune response, reflected by an elevated preoperative CRP in CC patients, has an independent prognostic value for disease-specific survival (DSS).
Patients and methods
The REMARK (Reporting Recommendations for Tumour Marker Prognostic Studies) criteria were applied [Citation7].
Patient data were collected retrospectively using case report formularies linked to an Access® database in each country. These databases were merged and converted for analyzes with IBM SPSS Statistics 20.0.
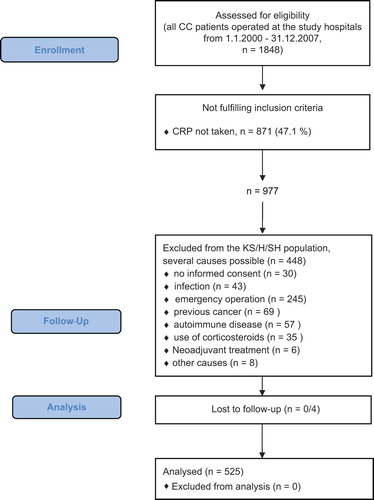
Data were obtained from national registers, local databases and/or patient records. All CC patients operated during the defined time period at the study hospitals were primarily included. The criteria applied for exclusion from the final analysis are depicted in .
Figure 1. Flow diagram of population-based inclusion from all CC patients with resected primary tumor from the study hospitals.
CRP was taken up to 20 days preoperatively and analyzed on the following platforms: Kristiansand/Norway: J & J Vitros 950 Chemistry Analyzer (Block Scientific, NY, USA).
Helsinki/Finland: Modular, Hitachi Ltd, Japan, Tokyo, Roche Diagnostics.
Stockholm/Sweden: Before 2003: Hitachi 917, Boehringer Mannheim; After 2003: Modular, Hitachi Ltd, Japan, Tokio, Roche Diagnostics. The total coefficient of variation (CV) for CRP assay on all platforms was estimated to be 4–6% at 10 and 70 mg/ml.
Patient characteristics
All patients had their primary tumors resected. It was registered if chemotherapy was given and if it was discontinued early due to toxicity. Fewer than 15% of stage IV patients received metastasectomy (data not shown). The primary end-point of the study was DSS, i.e. death due to colon cancer. Postoperative deaths (within 30 days) as well as deaths due to causes unrelated to CC were treated as competing events. Postoperative morbidity was defined to occur within 30 days and we analyzed the patient files for one of the following: thromboembolism, myocardial ischemia, wound infection, other infection, anastomotic leakage and postoperative treatment at an intensive care unit.
Statistics
Data were described with median and range (continuous variables) and with proportions and percentages (categorical variables). Crude associations between clinical and histopathology variables and CRP (categorized) were assessed with χ2-test and Fisher's exact test (when appropriate). Follow-up was defined as a time from the date of diagnosis to the date of death or study end, whichever came first. Death from CC was the main event for DSS and death of any other cause was the competing event. DSS was modeled using the competing risk approach [Citation8]. The results were expressed as sub-hazard rate ratios (SHR) with 95% confidence intervals (CI). P-values < 0.05 were considered statistically significant. All tests were two-sided. All analyzes were performed with IBM SPSS Statistics 20.0 and Stata ver 10.
Ethics
The study followed medical, ethical and legal guidelines for all participating countries (Finland, Norway and Sweden) and approvals were obtained from respective ethics committees. Confidentiality of patients included in the study was assured according to institutional guidelines of the hospitals and National Data Protection Commissions.
Results
In total, 1848 CC patients underwent a resection of their primary tumor at the study hospitals during the study period ().
In 977 (52.9%) of these patients CRP was taken preoperatively. Of these, 448 were excluded from the final analysis in accordance with the predefined exclusion criteria (see ). When comparing the patients with preoperative CRP available, with the patients with preoperative CRP not taken, patients with CRP available had a lower DSS (p = 0.013, data not shown). This difference could be ascribed to a significant difference in stage distribution towards higher stages (p = 0.017, data not shown) in the group where CRP was taken. Stage was highly associated with DSS (). Moreover, higher stage was associated with higher CRP values in our analysis (). In a multivariate Cox regression analysis adjusted for stage, there was no prognostic difference for CC death between the non-CRP and CRP groups (data not shown).
Table I. Patient characteristics and associations to CRP values. Associations between patient and tumor characteristics and the different CRP groups were assessed with the χ2-test, Fisher's exact test#; md, missing data; NA, not applicable, *determined with Kruskal-Wallis non-parametric ANOVA.
Table II. Factors possibly associated with decreased disease-specific survival in univariate and/or multivariate analysis. CI, confidence interval; NI, not included in the multivariate analysis; SHR, sub-hazard ratio; WHO grade, histologic differentiation grade. All analyses are performed using competing risk regression (Fine and Gray model).
In total, 525 patients from the three countries were included in the final analyzes. The following numbers of patients were analyzed from the different hospitals: Helsinki University Central Hospital in Helsinki/Finland from January 1998 to December 2007 (n = 61); Karolinska University Hospital in Huddinge, Stockholm/Sweden from January 2000 to December 2007 (n = 220); and Southern Hospital Trust in Kristiansand/Norway from January 2000 to December 2007 (n = 244).
Patient characteristics according to CRP values are shown in .
The median age was 73.0 years (range 31–97 years). Stage distribution was as follows: Stage I 77 (14.7%); Stage II 183 (34.8%); Stage III 145 (27.6%) and Stage IV 120 (22.9%) patients. Complete follow-up was available for all patients with a median of 4.5 years (range 0.0–14.4 years). Median CRP value for all patients was 10 mg/l (75th percentile 38 mg/l).
Age, TNM stage, WHO differentiation grade, right-sided tumor location all associated significantly with elevated CRP values. ASA status and postoperative morbidity were not associated with CRP levels. Postoperative morbidity also lacked association with CRP in multivariate analysis (logistic regression) ().
According to univariate analyzes, the following factors were prognostic for DSS in operated CC (): preoperative CRP, age, TNM stage, ASA status, WHO differentiation grade and postoperative morbidity. Higher levels of preoperative CRP and advanced TNM stage remained statistically significantly associated with higher risk of dying of CC also when adjusted for the above mentioned factors that were significant in univariate analyses. Patients with CRP > 30 were more than twice as likely to die from CC compared to patients with CRP < 10, [SHR = 2.1, 95% CI (1.39–3.10), ].
When assessing the possible association of CRP with postoperative morbidity in the multivariate logistic regression model, only ASA was found to independently associate with postoperative morbidity [ASA 0–2 vs. 3–4, HR 2.32, 95% (CI 1.49–3.62), p < 0.001] in a model adjusting also for stage, CRP (< 10, 10–30, > 30), and tumor site (data not shown).
The variables CEA (n = 282), albumin (n = 240), leukocyte count (n = 480) and BMI (n = 311) had many missing values, but were included and assessed in the multivariate model as well as a subset analysis. However, they were found not to be independent prognostic factors and were therefore omitted from the final model. Furthermore, CEA, albumin and leukocyte count showed significant association with CRP levels in the Kruskal-Wallis ANOVA analysis and with CRP in a Spearman Rank's correlation test (data not shown).
A raised preoperative CRP implies a poorer stage-specific postoperative prognosis in colon cancer
Finally, the prognostic significance of different CRP levels for DSS was determined for each stage ( and ).
Figure 2. Death due to colon cancer per stage. All analyses are performed using competing risk regression (Fine and Gray model).
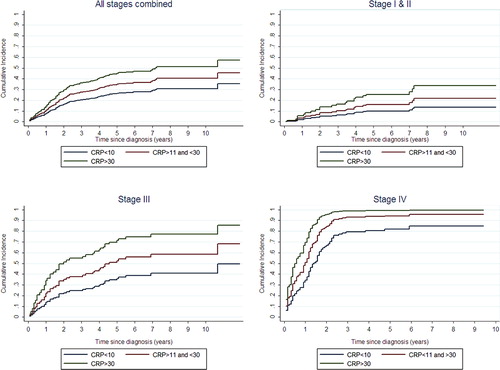
Table III. Factors associated with higher risk of dying of colon cancer. Uni- and multivariate competing risk regression, analyzed separately for each stage. CI, confidence interval; NI, not included in the multivariate analysis due to few patients with grade I tumors; SHR, sub-hazard ratio; WHO grade, histologic differentiation grade. All analyses are performed using competing risk regression (Fine and Gray model).
An elevated CRP was of prognostic significance for DSS in surgically treated CC patients regardless of stage. The cumulative incidences of CC-specific death are depicted in . Death of other causes was accounted for by means of competing risk analysis () depicts the cumulative incidence for all stages combined while depict cumulative incidences of CC death for each stage separately.
Due to the localized disease, Stages I and II patients are shown together in .
Elevated CRP levels were found to be a strong prognostic factor for DSS in CC. The risk of death due to CC augmented with increasing levels of CRP in every stage of operated CC. When analyzed separately for each stage, patients with CRP-levels above 60 mg/L were more than seven times more likely to die of CC compared to patients with CRP < 10 mg/L in the stages I and II subgroup of patients [SHR = 7.37 (CI 2.65–20.5)], more than three times more likely for stage III patients [SHR = 3.29 (CI 1.30–8.29)] and finally, more than twice as likely for patients with stage IV disease [SHR = 2.24 (CI 1.16–4.35)], for details see .
In a subset analysis, the prognostic value of CRP in stage III and IV patients was still significant, even when adjusted for chemotherapy given or not, data not shown.
Discussion
This Nordic multi-center study on patients operated on for CC is the largest analysis of the clinical significance of a preoperative SIR reported to date. An elevated CRP level was found to have strong prognostic significance in all stages of CC.
Importantly, the DSS for patients with high versus low CRP continued to diverge even after several years of follow-up. It seems that preoperative CRP elevation might reflect mechanisms other than acute infection, perforation or necrosis. A silent infection with a CRP over 30, despite of our strict exclusion criteria would be expected to lead to increased postoperative morbidity. However, postoperative morbidity was not increased in patients with an elevated preoperative CRP. These findings are in accordance with our hypothesis and the findings of others that elevated CRP levels may reflect a detrimental, pro-inflammatory immune response [Citation9].
The degree of CRP elevation was strongly associated with right-sided tumor location. This cannot be explained by advanced tumor since there was no difference in the TNM-stage distribution in relation to tumor location, in the present (data not shown) nor in a previous study [Citation10]. One might speculate that the greater chance of subclinical bowel obstruction in left-sided tumors should lead to a greater proportion of patients with elevated CRP values. In this cohort, however, the reverse association was seen.
It is noteworthy, that proximal bowel tumors have a higher frequency of microsatellite instability (MSI-high) than those in the distal bowel [Citation11]. Amongst the effects of the drug acetylsalicylic acid (Aspirin) is to block cyto- and chemokines that recruit inflammatory cells, that in turn facilitate cancer immune escape [Citation12]. In fact acetylsalicylic acid has repeatedly been shown to be effective in the prevention of proximal and MSI-high phenotype CCs but interestingly not rectal cancer [Citation13].
The present study has several strengths.
First, our study is large and population based, hence our results can be generalized to an unselected Nordic population of colon cancer patients.
Second, long-term follow-up data are presented with DSS.
Third, since rectal cancers have been reported to be associated with lower CRP values [Citation6], the present study analyzed CC patients only, whereas this has not been the case in previous studies [Citation14–17].
Fourth, because of the smaller materials in single-center studies, the prognostic significance of CRP either has been determined only in selected stages [Citation14–16] or has not been analyzed by stage [Citation17]. In our international multicenter study, the prognostic significance of elevated preoperative CRP could be assessed for each stage separately due to the relatively large number of patients. However, the present study is in concordance regarding the increase in CRP with advancing stage and the prognostic significance of increasing CRP as a continuous variable.
Due to the retrospective nature of our study, there are several important weaknesses and therefore, the results should be interpreted as hypothesis generating. Since we had no control over the exposure of treatments this may be a potential bias, especially in stage IV patients. We only recorded if chemotherapy was given and if it was terminated early due to toxicity. However, type of chemotherapy, treatment holidays, etc. may be confounding factors.
Another disadvantage of this retrospective study is that not all variables were available in a sufficient proportion of patients to allow comparison of their prognostic significance with regard to CRP. Others, however, have suggested the superiority of CRP as an inflammatory prognostic marker [Citation6,Citation18]. Furthermore one should bear in mind that these laboratory tests may have been performed for a particular clinical reason, which may have biased the results.
Several recent reports from single-center studies combine albumin levels with CRP in the analysis of SIR in CC [Citation17,Citation19]. The combination of CRP and albumin has been termed the Glasgow prognostic scale (GPS). However, the GPS was developed for lung cancer [Citation20] and, to the best of our knowledge, it has not been demonstrated that albumin adds independent prognostic value to CRP in CC. Unfortunately, we had albumin values available only in 240 of the 525 patients of our dataset. Since albumin may have been analyzed for specific reasons in these patients, any analysis of albumin's significance should be treated with caution. We found albumin to correlates strongly with CRP in our dataset, thus adding little prognostic value to CRP. This is in line with the conclusions of other studies [Citation21].
The prognostic significance of preoperatively elevated CRP in CC leads to the question: what biological mechanisms cause SIR with elevated CRP? This is an important topic for further research as well as the question: how can this knowledge be implemented in clinical practice?
In line with the important prognostic histopathologic description of the beneficial immune response [Citation22], the potential of using the immune system in treating cancer patients has regained popularity after the introduction of T-cell checkpoint inhibitors. However, Rudolph Virchow and others have described the immune system as a double-edged sword with beneficial as well as tumor-promoting effects [Citation23]. The existence of a SIR could potentially be important in stratifying patients in immunotherapy protocols. In melanoma patients, it has been suggested, that patients with elevated CRP may have a detrimental outcome after immunotherapy, whereas those with normal CRP values seem to benefit from immunotherapy [Citation24]. In that respect, the association of SIR with prognosis in the study of COX-2 inhibitors gains additional importance. Randomized studies on the effect of acetylsalicylic acid in the adjuvant setting in CC patients are ongoing [Citation25] and we suggest a subanalysis of groups according to degree of CRP elevation. Future randomized trials on acetylsalicylic acid or COX-2 inhibitors should, in our opinion, be stratified according to the degree of SIR.
Most Stage III CC patients are over-treated with adjuvant chemotherapy, whereas some Stage II patients are under-treated. Several different gene expression profiles have been suggested to be of clinical value in selecting patients for adjuvant therapy. However, the complexity and cost of these tests, as well as their limited clinical value has thus far precluded their widespread routine use in clinical practice. Here, we report on a readily available blood test for the price of $15.00 that exhibits comparable prognostic value. Thus, stratification according to CRP values before randomization, in surgical as well as in drug development trials, appears indicated for future trials. Prospective larger scale studies are now warranted to confirm and extend the findings of the present work.
Conclusion
An elevated preoperative CRP is associated with poorer postoperative disease-specific prognosis in all stages of CC. Inherent pro-tumorigenic inflammatory mechanisms should be investigated in translational studies. In clinical studies analyzing the potential of chemo- or immunotherapy, patients should be stratified according to CRP value to reveal the predictive value of CRP.
Acknowledgments
CK has received a general research grant from the Southern Hospital Trust. JL has been awarded a Fellowship grant from Sigrid Juselius Foundation and a Research grant from The Finnish Medical Foundation. Collaboration between the authors of this group was initiated and facilitated within the framework of the GI oncology 2010 course and the authors express their sincere gratitude to the organizers of this course. Establishment of the clinical databases was made possible through the meticulous work and enduring help of Bente Mirjam Christensen, Margareta Michanek, Elina Aspiala and Thyra Löwenmark.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Patama TEG, Klint Å, Larønningen S, Ólafsdóttir GH, Pukkala E. Small-area based map animations of cancer mortality in the Nordic countries, 1971–2003. Cancer Union 2008. Available from: http://astracancerfi/cancermaps/ Nordic/mort [cited 2013 June 15].
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J clin oncol 2011;29:610–8.
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63.
- Chung YC, Chang YF. Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. Eur J Gastroenterol Hepatol 2003;15:369–73.
- Roxburgh CS, Salmond JM, Horgan PG, Oien KA, McMillan DC. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer 2009;45:2138–45.
- Nielsen HJ, Christensen IJ, Sorensen S, Moesgaard F, Brunner N. Preoperative plasma plasminogen activator inhibitor type-1 and serum C-reactive protein levels in patients with colorectal cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol 2000;7:617–23.
- Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and elaboration. PLoS Med 2012;9:e1001216.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94:496–509.
- Roxburgh CS, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 2012;38:451–66.
- Crozier JE, McMillan DC, McArdle CS, Angerson WJ, Anderson JH, Horgan PG, et al. Tumor size is associated with the systemic inflammatory response but not survival in patients with primary operable colorectal cancer. J Gastroenterol Hepatol 2007;22:2288–91.
- Wong JJ, Hawkins NJ, Ward RL, Hitchins MP. Methylation of the 3p22 region encompassing MLH1 is representative of the CpG island methylator phenotype in colorectal cancer. Mod Pathol 2011;24:396–411.
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883–99.
- Bastiaannet E, Sampieri K, Dekkers OM, de Craen AJ, van Herk-Sukel MP, Lemmens V, et al. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer 2012;106:1564–70.
- Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, et al. Preoperative C-reactive protein as a prognostic and therapeutic marker for colorectal cancer. J Surg Oncol 2008; 98:540–4.
- Sugimoto K, Komiyama H, Kojima Y, Goto M, Tomiki Y, Sakamoto K. Glasgow prognostic score as a prognostic factor in patients undergoing curative surgery for colorectal cancer. Dig Surg 2013;29:503–9.
- Bystrom P, Berglund A, Nygren P, Wernroth L, Johansson B, Larsson A, et al. Evaluation of predictive markers for patients with advanced colorectal cancer. Acta Oncol 2012;51:849–59.
- Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Kubota K. Inflammation-based prognostic system predicts postoperative survival of colorectal cancer patients with a normal preoperative serum level of carcinoembryonic antigen. Ann Surg Oncol 2012;19:3422–31.
- Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O’Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer 2011;47:2633–41.
- Roxburgh CS, Platt JJ, Leitch EF, Kinsella J, Horgan PG, McMillan DC. Relationship between preoperative comorbidity, systemic inflammatory response, and survival in patients undergoing curative resection for colorectal cancer. Ann Surg Oncol 2011;18:997–1005.
- Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer 2003; 89:1028–30.
- Crumley AB, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer?. World J Surg 2010;34:2393–8.
- Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011;71:5601–5.
- Eggermont AM. Therapeutic vaccines in solid tumours: Can they be harmful?. Eur J Cancer 2009;45:2087–90.
- Marhall MA, Ribas A, Huang B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J Clin Oncol 2010;28(Suppl 2609). (Meeting Abstracts).
- Ali R, Toh HC, Chia WK, Investigators AT. The utility of aspirin in Dukes C and high risk Dukes B colorectal cancer – the ASCOLT study: Study protocol for a randomized controlled trial. Trials 2011;12:261.