Abstract
Background and aims. Platelets are believed to promote tumor growth and metastasis but their prognostic role in locally advanced pancreatic cancer (LAPC) remains largely unknown. We assessed whether pretreatment platelet counts independently predict survival outcomes in patients with LAPC treated with chemoradiation (CRT).
Methods. We retrospectively reviewed the MD Anderson pancreatic cancer database and identified 199 patients with LAPC treated with CRT between 2006 and 2012. Induction chemotherapy was used prior to consolidative CRT in 177 (89%) patients. Median radiation dose was 50.4 Gy. Concurrent radiosensitizers were gemcitabine-based (13%) or capecitabine-based (84%) regimens. Actuarial univariate and multivariate statistical methods were used to determine significant prognostic factors for overall survival (OS) and progression-free survival (PFS) calculated from the start of treatment.
Results. Median follow-up was 9.9 months. Median OS and PFS durations were 17.7 and 10.7 months, respectively. On univariate analysis, platelet count > 300 K/μl, KPS ≤ 80, ≥ 5% weight loss and pretreatment CA19-9 above the median were associated with inferior OS or PFS. Median OS was lower in patients with platelet count > 300 K/μl compared to patients with platelet count ≤ 300 K/μl (10.2 vs. 19 months; p = 0.0002). Corresponding median PFS times were 7.8 months and 11.1 months (p = 0.004), respectively. On multivariate analysis, platelet count > 300 K/μl (p = 0.012), ≥ 5% weight loss (p = 0.002) and elevated pretreatment CA19-9 (p = 0.005) were independent prognostic factors for OS. Platelet count > 300 K/μl (p = 0.03) and KPS ≤ 80 (p = 0.05) independently predicted PFS.
Conclusions. Our analysis suggests that pretreatment thrombocytosis independently predicts inferior OS and PFS in LAPC.
Cancer-associated thromboss, first described by Armand Trousseau in 1865, has long been considered an epiphenomenon of cancer progression [Citation1]. In fact, high circulating platelet counts have been associated with an unfavorable prognosis in a number of malignancies, including ovarian, renal and gastric cancers [Citation2–4]. These observations have led to extensive research to explore the functions of platelets beyond hemostasis. Recent studies show that platelets promote tumor growth and progression by regulating and maintaining angiogenesis [Citation5–7]. In addition, P-selectin (a vascular cell adhesion molecule expressed on platelets) mediates platelet-tumor cell interactions that have been shown to enhance metastatic dissemination by assisting vascular invasion and immune-evasion [Citation5,Citation7]. The clinical correlation between platelet dysfunction and tumor progression is further supported by findings in mouse models where experimentally induced thrombocytopenia reduces tumor growth and attenuates metastasis [Citation8].
Although the clinical observation of migratory thrombosis has classically been associated with gastrointestinal (GI) malignancies, very few studies have addressed the prognostic significance of high platelet counts in this population [Citation3,Citation9,Citation10]. Pancreatic cancer is the third most common GI malignancy and one of the most aggressive tumors to treat. With annual incidence and mortality rates mirroring one another, it is the fourth leading cause of cancer-related death in the US [Citation11]. About 50% of all pancreatic cancer patients present with radiographically detectable metastases, which in part explains the high mortality rate associated with the disease. Margin-negative surgical resection offers the only means of cure but unfortunately, only 10–15% of patients present with tumors amenable to resection [Citation12]. The remaining patients have locally advanced disease defined as non-metastatic, but surgically unresectable or borderline resectable disease where there is the possibility of resectability if there is a favorable response to treatment [Citation13]. Despite some recent therapeutic advances, the prognosis of patients with locally advanced unresectable pancreatic cancer (LAPC) remains largely dismal with a median survival of 9–13 months [Citation14]. The standard treatment for LAPC in the US consists of a combination of chemotherapy and radiation therapy, but considerable variation exists in the integration of these modalities and the respective drug regimens and dose schedules.
The prognostic factors for pancreatic cancer described in the literature include performance status, weight loss, pretreatment hemoglobin level, carbohydrate antigen 19-9 (CA19-9) level, clinical stage at presentation and the treatment modality [Citation15–19]. However, the prognostic significance of platelet counts in patients with pancreatic cancer remains largely unknown. This study was undertaken to identify the potential prognostic significance of pretreatment platelet counts in a large cohort of patients with LAPC treated with a combination of chemotherapy and radiation therapy. We also looked at the prognostic factors for LAPC found to be previously significant in our studies.
Patients and methods
Patient identification and selection
We conducted a retrospective consecutive cohort study of patients with biopsy-proven, LAPC treated with chemoradiation (CRT) at our institution between 2006 and 2012. Patients were selected from our institutional pancreatic cancer database and tumor registry. A tumor was deemed locally advanced if it extended to the celiac axis, the superior mesenteric artery (> 180° encasement) or the aorta or occluded the superior mesenteric-portal venous confluence, based on review of computerized tomography (CT) images. We excluded cases with either primarily resectable disease or borderline resectable status (as per the MD Anderson classification) or distant metastasis at the time of diagnosis [Citation13]. All cases were evaluated by a multidisciplinary team including a medical oncologist and a radiation oncologist and selected patients were seen by surgical oncologists. The Institutional Review Board of The University of Texas M D Anderson Cancer Center approved the study.
Patient characteristics
All patients with suspected LAPC underwent complete history and physical examination, a dedicated pancreatic cancer protocol contrast-enhanced dual phase abdominal and pelvic CT scan and biopsy (for histological confirmation). Pretreatment assessment also included laboratory tests like complete blood count, blood electrolytes, urea, creatinine, liver transaminases, alkaline phosphatase, CA19-9 and total bilirubin. Platelet counts were recorded preferably on the day of start of treatment (induction chemotherapy or definitive CRT). If no counts were available on the day of start of treatment, the most recent pre-treatment value was used as long as it was within one month of start of treatment. Karnofsky performance status (KPS) and weight loss were prospectively recorded by the treating physician(s) prior to therapy.
Treatment characteristics
The initial treatment modality consisted of either systemic chemotherapy before chemoradiation (CCR) or only CRT. This was based on physician preference. FOLFIRINOX or gemcitabine-based induction chemotherapy was used for patients receiving the CCR modality. The median radiation dose administered was 50.4 Gy concurrent with gemcitabine-based or capecitabine-based concurrent chemotherapy. In a few cases, protocol-based concurrent therapy included the addition of vorinostat, or bevacizumab and erlotinib. Radiation therapy was administered using megavoltage x-rays using either a four-field three-dimensional conformal technique or an intensity-modulated radiation therapy (IMRT) technique.
Follow-up
Follow-up visits with a medical or radiation oncologist were initially scheduled for all patients at one month (after completion of CRT) and then every 3–4 months. Disease restaging was done by abdominal-pelvic CT scans and chest x-rays during these visits. Subsequent therapy was individualized based on the presence of disease progression. Cases where adequate downstaging had occurred were considered for surgical resection.
Prognostic factors
Prognostic factors were chosen based on the previously published data from our group as well as other studies [Citation15–17]. Data analyzed included demographic, patient-, tumor- and treatment-specific variables. Demographic variables included age, gender, and ethnicity. Patient-specific variables analyzed included pretreatment platelet count (analyzed as a continuous variable and by dichotomizing at 300 K/μl), pretreatment hemoglobin (analyzed as a continuous variable and by dichotomizing at 12 g/dl), pretreatment Ca 19-9 (after excluding cases with values < 50 U/ml and bilirubin > 2 mg/dl; analyzed as a continuous variable and by dichotomizing at the median value), Karnofsky Performance Status (KPS > 80 vs. KPS ≤ 80) and percentage weight loss (≥ 5% vs. < 5% in three months prior to presentation). Tumor-specific variables analyzed included type of histology (adenocarcinomas vs. variants, such as adenosquamous, signet ring and mucinous carcinoma) and histological grade (where available). Treatment-specific factors analyzed included type of systemic chemotherapy used for induction, concurrent chemotherapy and the treatment modality (CCR vs. CRT).
End points
The major end points were overall survival (OS) and progression-free survival (PFS). In addition, we also analyzed local-regional recurrence (defined as any recurrence at or adjacent to the initial primary site or in the regional lymph nodes as determined by abdominal-pelvic CT scans), and distant recurrence (defined by the presence of malignant ascites or metastasis to the peritoneum, liver, lung, bone or any other distant site).
Statistical analysis
OS time was calculated from the start of treatment (induction chemotherapy for CCR group and radiation therapy for CRT group) to the time to death or censorship (time of the last follow-up on record if death was not observed). PFS time was censored at the date of the last follow-up on record if no recurrence (local-regional or distant) or death was observed. Local-regional failure and distant failure times were calculated from the start of treatment to the date of local-regional and distant recurrence, respectively. Categorical data were summarized by frequency and comparisons were performed using χ2-test for proportions. The means between the groups were compared using Student's t-test. Kaplan-Meier's product limit method was used to non-parametrically estimate the survival probabilities and the difference between groups was compared using log-rank test. Prospectively selected prognostic factors were investigated by univariate analysis. A Cox proportional hazards model was constructed for multivariate comparisons using variables identified as statistically significant on univariate analysis. All tests were two-sided and a p-value of ≤ 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 199 patients were diagnosed and treated for LAPC with CRT during the study period. Pretreatment platelet counts were available for 180 patients. The median age at the time of diagnosis was 64 years (range 37–88 years) and majority were men (56%). The median platelet count prior to any form of treatment was 221 K/μl (range 85–643) and 32 (18%) had counts more than 300 K/μl. The median hemoglobin was 12.8 g/dl (range 9–18) and median pretreatment CA19-9 was 332 U/ml. Hemoglobin levels did not differ significantly between patients with platelet counts more than 300 K/μl and ≤ 300 K/μl (12.5 vs. 12.9 g/dl; p = 0.17). In addition, the mean corpuscular volume in patients with platelet counts more than 300K/μl was 89 fL (SD, 7.1), suggesting that iron deficiency anemia was unlikely to be responsible for the high platelet count. summarizes the patient characteristics.
Table I. Patient characteristics.
Tumor and treatment characteristics
The median tumor size was 3.9 cm (range 1–8) in the largest dimension. The majority of tumors were adenocarcinomas (96%), located mainly in the head of the pancreas (63%). The histological grade was available for only 64 cases and most of them were moderately differentiated (52%). Of the total cohort, 177 (89%) patients received systemic chemotherapy before chemoradiation (CCR) and 22 (11%) received only CRT as their primary treatment modality. The median time for initiation of treatment was 23 days (range 1–148 days) from the date of diagnosis. The systemic chemotherapy was administered for a median duration of 3 months (range 1–11 months) using FOLFIRINOX (n = 28, 16%), gemcitabine-based (n = 147, 83%) or capecitabine-based (n = 2, 1%) therapy. The median dose of radiation was 50.4 Gy (range, 25.2–70.4 Gy). Concurrent chemotherapy included 5-fluorouracil (n = 4, 2%), vorinostat (n = 1, 1%), gemcitabine-based (n = 26, 13%) or capecitabine-based (n = 167, 84%) therapy. The treatment regimen was similar between patients with platelet count ≤ 300 K/μl and those with > 300 K/μl. Both groups received a median radiation dose of 50.4 Gy in 28 fractions. In patients with platelet count ≤ 300 K/μl, 128 (86%) patients received concurrent capecitabine while 17 (11%) received gemcitabine. However, in patients with platelet count > 300 K/μl, 26 (81%) were treated with concurrent capecitabine while five (16%) received gemcitabine. The median clinical follow-up time was 9.9 months (range 1–86) and radiological follow-up time was 9.1 months (range 1–85). Following CRT, 11 patients were able to undergo surgical resection of their pancreatic tumor, initially deemed unresectable at the time of diagnosis. summarizes tumor and treatment characteristics.
Table II. Tumor and treatment characteristics.
Recurrence and survival analysis
A total of 172 patients had died at the time of analysis. The median actuarial OS was 17.7 months (range 1–89 months). Estimated rate of OS at the end of one, two and three years were 69%, 29% and 15%, respectively. Median PFS was 10.7 months (range 1–89 months). Median time for local recurrence was 15.3 months (range 1–89 months) and distant recurrence was 17.4 months (range 1–89 months). Patients with platelet count more than 300 K/μl had an inferior OS (10.2 vs. 19 months, p = 0.0002; ) and PFS (7.8 vs. 11.1 months, p = 0.004; ) as compared to those with platelets ≤ 300 K/μl. Although a similar association was observed with platelet counts more than 350 K/μl, 400 K/μl and 450 K/μl, the sample size for these groups was underpowered to detect a significant difference. On univariate analysis, other significant prognostic factors for inferior OS were KPS ≤ 80 (p = 0.018), ≥ 5% weight loss in the preceding three months (p = 0.001) and baseline CA19-9 greater than the median value (p = 0.05). The other significant prognostic factor for inferior PFS was ≥ 5% weight loss in the preceding three months (p = 0.02) and KPS ≤ 80 (p = 0.04). summarizes the results of the univariate analysis. The results for OS and PFS did not alter after excluding patients who had non-adenocarcinoma as the presenting histology (n = 8).
Figure 1. Kaplan-Meier estimates of overall survival in patients with locally advanced pancreatic cancer according to pretreatment platelet levels.
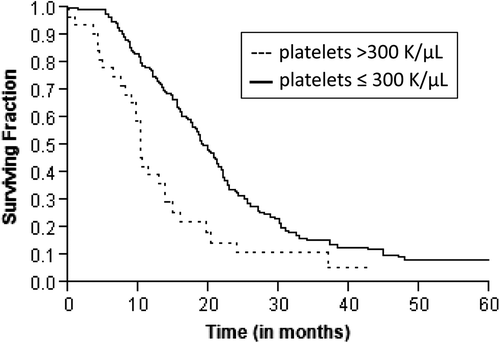
Figure 2. Kaplan-Meier estimates of progression-free survival in patients with locally advanced pancreatic cancer according to pretreatment platelet levels.
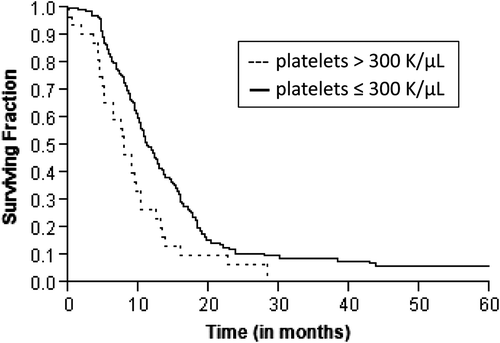
Table III. Univariate analyses of prognostic factors for outcomes.
Multivariate analysis
On multivariate analysis, pretreatment platelet count more than 300 K/μl (HR = 2.22, p = 0.012), pretreatment CA19-9 above the median (HR = 1.87, p = 0.005) and ≥ 5% weight loss (HR = 2.27, p = 0.002) were independent predictors of OS. For PFS, pretreatment platelet count more than 300 K/μl (HR = 1.73, p = 0.03) and KPS ≤ 80 (HR = 1.43, p = 0.05) were the independent prognostic factors. summarizes the results of the multivariate analysis.
Table IV. Multivariate analyses of prognostic factors for outcomes.
Discussion
Our study shows that high pretreatment platelet counts (> 300 K/μl) independently predict inferior OS and PFS in patients with unresectable pancreatic cancer. Apart from platelets, percentage weight loss and elevated baseline CA19-9 are independent predictors of OS while KPS independently predicts PFS.
Though pancreatic cancers have traditionally been associated with hypercoagulable states, the prognostic and predictive significance of pretreatment platelet counts in pancreatic cancer is largely unknown. One study had previously investigated the role of platelets in predicting survival following resection in periampullary carcinomas [Citation10]. However, it included patients with duodenal and bile duct cancers in addition to surgically resectable pancreatic tumors. To the best of our knowledge, this study is the first to examine the prognostic significance of pretreatment platelets in an exclusive set of patients with unresectable LAPC treated with CRT. Our findings are in accordance with previously published studies on paraneoplastic thrombocytosis in ovarian, renal and gastric cancer [Citation2–4]. The association between weight loss, elevated baseline CA19-9 and prognosis is also congruent with previously published data [Citation19,Citation20]. Performance status is one of the most recognized prognostic factors shown to influence OS in pancreatic cancer [Citation15,Citation20]. Although KPS was shown to predict PFS and OS on univariate analysis, its effect on OS was subsequently overshadowed by other parameters in the multivariate proportional hazards model. Interestingly, pretreatment hemoglobin > 12 g/dl which was previously shown to be associated with improved OS by our group did not show significance in this cohort.
Two potential theories could account for the observed detrimental effect of thrombocytosis on treatment outcomes with CRT therapy. One possibility is that elevated platelet counts contribute to sluggish blood flow and microemboli formation within the tumor microvasculature. This, in turn, leads to tissue hypoxia which is an established mediator of radioresistance [Citation21–23]. The other possibility is that elevated platelet counts promote tumor growth. Although the association between thrombosis in cancer patients and poor clinical outcomes has been validated in a number of clinical and epidemiological studies, the recognition of a two-way link between tumor-cell-platelet interactions is more recent. This is based on studies in mouse models which show that platelets actively contribute to tumor growth and metastasis through a paracrine circuit involving Interleukin 6 (IL-6) and thrombopoietin and their presence is not merely an epiphenomenon [Citation2]. So, on one hand, tumors secrete IL-6 and this leads to thrombopoietin synthesis in the liver which, in turn, leads to enhanced synthesis of platelets in the bone marrow. On the other hand, mounting evidence indicates that platelets exert a direct stimulatory role in promoting angiogenesis, a critical step in the evolution of tumors from a benign state to a malignant one and an essential step in permitting invading tumor cells access to the systemic circulation for metastatic dissemination [Citation5–7,Citation24,Citation25]. The secretory granules of platelets contain many proangiogenic factors like vascular endothelial growth factor, epidermal growth factors and platelet-derived growth factor and their concentrations are found to increase in the presence of a tumor [Citation5,Citation7]. Recent findings also suggest that platelets may stabilize tumor microvasculature via their secretory activity and prevent intratumoral hemorrhage [Citation26]. Platelets also modulate basement membrane and extracellular matrix proteolysis; directly by releasing proteinases and indirectly via causing endothelial cells and tumor cells to release proteolytic enzymes, thereby promoting angiogenesis and tumor invasion. Thus a feed forward loop is created wherein tumors stimulate production of platelets (via IL-6 and thrombopoietin) and the increased platelet counts, in turn, promote tumor growth and metastasis (via stimulation of invasion and angiogenesis) which further increases the tumor-derived IL-6 level production [Citation2,Citation27–29]. The indispensable role of platelets in establishing this circuit is further supported by provisional data generated by The Cancer Genome Atlas Research Network which shows that the MPL gene expression (for the thrombopoietin receptor) is up regulated in only 5% of pancreatic adenocarcinomas [Citation30,Citation31]. In addition, studies in solid tumors, including pancreatic cancer, also show low or no expression of thrombopoietin receptor on the tumor surface which suggests that tumor-derived thrombopoietin synthesis by itself is insufficient to complete the paracrine loop [Citation32,Citation33]. Taken together, high platelet counts may thus portend an inferior prognosis not only by making tumors more treatment resistant but also by contributing to tumor growth and metastasis.
From a clinical management point of view, platelets offer an exciting target for therapeutic intervention, but their multifunctional role in hemostasis and tumor growth/treatment resistance makes it challenging [Citation5]. Prospective clinical trials show a survival benefit with low-molecular-weight heparin among patients with cancer, independent of its protective role against vascular thromboembolic complications [Citation34]. Similarly, antiplatelet agents may also have some therapeutic value but this often comes with the associated risk of bleeding complications and may not be advisable for all patients with high platelet counts. Furthermore, this does not directly address the issue of increased platelet production via tumor-induced cytokines.
Chemotherapeutic agents often lead to thrombocytopenia. In our study, 69% of the patients (CCR group) had a decrease in platelet count from their pretreatment value following induction chemotherapy. This is typically a rapid drop in the platelet count that predates any measurable anti-tumor effect, suggesting that the root cause of this decline in platelet counts is due to bone marrow suppression rather than reduction in the production of thrombogenic tumor-derived factors (like IL-6). Notably, the chemotherapy-induced decrease in platelet counts did not confer any survival advantage to that cohort of patients. This suggests that thrombocytosis present at the beginning of therapy is likely a paraneoplastic phenomenon rather than an unrelated extraneous or excessive production of a tumor growth enhancing bystander. In thinking of high platelet count itself as a modifiable risk factor for pancreatic cancer, rather than intervening at the level of platelet production and/or clot formation, we believe that it may be more advantageous to target intermediates along the paracrine signaling pathway originating at the level of the tumor (like IL-6) and/or the liver (thrombopoietin). In principle, this would address the root cause of paraneoplastic thrombocytosis and at the same time, preserve the platelet aggregation and adhesion functions necessary for hemostasis.
As with most retrospective analyses, this study should be interpreted in the context of several limitations. First, our data cannot be generalized to patients with obvious metastases or those with resectable disease. Second, the retrospective approach of this study renders analyses of patient- and treatment-related variables exploratory, thereby providing means to only generate testable hypotheses. We cannot infer whether the high platelet count is simply a host response to the advanced tumor or is a contributing factor to tumor progression. Third, we only included patients who had pretreatment platelet counts available, possibly introducing a selection bias. Furthermore, the retrospective nature of this study necessitated the use of defining a time constraint of one month for recording pretreatment platelet counts. This lead to some variability in the timing of collection of pretreatment platelet counts relative to the start of treatment. Finally, the changes in imaging quality and radiation therapy techniques during the period of seven years may have introduced unknown confounding factors.
The major strength of our study is the relatively large sample size. It is also the first study to examine whether pretreatment platelet counts influence disease outcome in this selective group of patients with LAPC treated with CRT therapy. The treatment criteria and the treatment modality were relatively consistent throughout the study period. The multidisciplinary management involving a dedicated team of clinicians from various fields specializing in pancreatic cancer is also an asset to such an investigation.
In conclusion, our data suggests that high pretreatment platelet count is an independent prognostic factor for patients with LAPC treated with CRT. This finding expands the steadily growing evidence of tumor-platelet cross-talk to unresectable pancreatic cancer. These results provide rationale to design prospective studies to investigate the role of antiangiogenic agents like IL-6 inhibitors in pancreatic cancer and to test whether high platelet counts contribute to radioresistance in an experimental setting.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Trousseau A. Lectures on clinical medicine, delivered at the Hotel-Dieu, Paris. 1865;5:281–332.
- Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. New Engl J Med 2012;366:610–8.
- Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 2002;9:287–91.
- Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. Br J Urol Int 2000;86:203–7.
- Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther 2008;8:1247–55.
- Sierko E, Wojtukiewicz MZ. Platelets and angiogenesis in malignancy. Semin Thromb Hemost 2004;30:95–108.
- Caine GJ, Lip GY, Blann AD. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: Further evidence for a role of platelets in tumour angiogenesis. Ann Med 2004;36:273–7.
- Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest 1988;81:1012–9.
- Aminian A, Karimian F, Mirsharifi R, Alibakhshi A, Dashti H, Jahangiri Y, et al. Significance of platelet count in esophageal carcinomas. Saudi J Gastroenterol 2011;17:134–7.
- Schwarz RE, Keny H. Preoperative platelet count predicts survival after resection of periampullary adenocarcinoma. Hepatogastroenterology 2001;48:1493–8.
- Willett CG, Czito BG, Bendell JC, Ryan DP. Locally advanced pancreatic cancer. J Clin Oncol 2005;23: 4538–44.
- Gutt R, Liauw SL, Weichselbaum RR. The role of radiotherapy in locally advanced pancreatic carcinoma. Nature Rev Gastroenterol Hepatol 2010;7:437–47.
- Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg 2008;206:833–46; discussion 46–8.
- Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer. Ann Oncol 2013;24:2484–92.
- Krishnan S, Rana V, Janjan NA, Abbruzzese JL, Gould MS, Das P, et al. Prognostic factors in patients with unresectable locally advanced pancreatic adenocarcinoma treated with chemoradiation. Cancer 2006;107:2589–96.
- Ikeda M, Okada S, Tokuuye K, Ueno H, Okusaka T. Prognostic factors in patients with locally advanced pancreatic carcinoma receiving chemoradiotherapy. Cancer 2001; 91:490–5.
- Sezgin C, Karabulut B, Uslu R, Sanli UA, Goksel G, Yuzer Y, et al. Gemcitabine treatment in patients with inoperable locally advanced/metastatic pancreatic cancer and prognostic factors. Scand J Gastroenterol 2005;40:1486–92.
- Krishnan S, Rana V, Janjan NA, Varadhachary GR, Abbruzzese JL, Das P, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47–55.
- Vainshtein JM, Schipper M, Zalupski MM, Lawrence TS, Abrams R, Francis IR, et al. Prognostic significance of carbohydrate antigen 19–9 in unresectable locally advanced pancreatic cancer treated with dose-escalated intensity modulated radiation therapy and concurrent full-dose gemcitabine: Analysis of a prospective phase dose escalation study. Int J Radiat Oncol Biol Phys 2013;86:96–101.
- Tas F, Sen F, Odabas H, Kilic L, Keskin S, Yildiz I. Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol 2013; 18:839–46.
- Sugrue T, Lowndes NF, Ceredig R. Hypoxia enhances the radioresistance of mouse mesenchymal stromal cells. Stem Cells 2014;32:2188–200.
- Chan N, Koritzinsky M, Zhao H, Bindra R, Glazer PM, Powell S, et al. Chronic hypoxia decreases synthesis of homologous recombination proteins to offset chemoresistance and radioresistance. Cancer Res 2008;68:605–14.
- Schack JA, Macduffee RC. Increased radioresistance of red bone marrow after anoxia. Science 1949;110:259–60.
- Wojtukiewicz MZ, Sierko E, Rak J. Contribution of the hemostatic system to angiogenesis in cancer. Semin Thromb Hemost 2004;30:5–20.
- Folkman J, Browder T, Palmblad J. Angiogenesis research: Guidelines for translation to clinical application. Thromb Haemost 2001;8:23–33.
- Ho-Tin-Noe B, Goerge T, Wagner DD. Platelets: Guardians of tumor vasculature. Cancer Res 2009;69:5623–6.
- Wojtukiewicz MZ, Sierko E, Klement P, Rak J. The hemostatic system and angiogenesis in malignancy. Neoplasia (New York, NY). 2001;3:371–84.
- Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature 1997;386:616–9.
- Belloc C, Lu H, Soria C, Fridman R, Legrand Y, Menashi S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int J Cancer 1995;60:413–7.
- Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1.
- Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4.
- Graf G, Dehmel U, Drexler HG. Expression of thrombopoietin and thrombopoietin receptor MPL in human leukemia-lymphoma and solid tumor cell lines. Leukemia Res 1996; 20:831–8.
- Columbyova L, Loda M, Scadden DT. Thrombopoietin receptor expression in human cancer cell lines and primary tissues. Cancer Res 1995;55:3509–12.
- Akl EA, van Doormaal FF, Barba M, Kamath G, Kim SY, Kuipers S, et al. Parenteral anticoagulation for prolonging survival in patients with cancer who have no other indication for anticoagulation. Cochrane Database Syst Rev 2007;(3): CD006652.
