Abstract
Background. In metastatic colorectal cancer, mutation testing for KRAS exon 2 is widely implemented to select patients with wild-type tumors for treatment with the monocloncal anti-EGFR antibodies cetuximab and panitumumab. The added predictive value of additional biomarkers in the RAS-RAF-MAPK and PI3K-AKT-mTOR pathways in colorectal cancer is uncertain, which led us to systematically review the impact of alterations in KRAS (outside of exon 2), NRAS, BRAF, PIK3CA and PTEN in relation to the clinical benefit from anti-EGFR treatment.
Methods. In total, 22 studies that include 2395 patients formed the basis for a meta-analysis on alterations in KRAS exons 3 and 4, NRAS, BRAF, and PIK3CA and PTEN and outcome of anti-EGFR treatment. Odds ratios for objective response rate (ORR) and hazard ratios (HR) for progression-free survival (PFS) and overall survival (OS) were calculated.
Results. Mutations in KRAS exons 3 and 4, BRAF, PIK3CA and non-functional PTEN (mutations or loss of protein expression) significantly predicted poor ORR (OR = 0.26, OR = 0.29, OR = 0.39, and OR = 0.41, respectively). Significantly shorter PFS applied to mutations in KRAS exons 3 and 4 (HR = 2.19), NRAS (HR = 2.30) and BRAF (HR = 2.95) and non-functional PTEN (HR = 1.88). Significantly shorter OS applied to mutations in KRAS exons 3 and 4 (HR = 1.78), NRAS (HR = 1.85), BRAF (HR = 2.52), PIK3CA (HR = 1.43) and alterations in PTEN (HR = 2.09).
Conclusions. Meta-analysis suggests that mutations in KRAS exons 3 and 4, NRAS, BRAF and PIK3CA and non-functional PTEN predict resistance to anti-EGFR therapies and demonstrates that biomarker analysis beyond KRAS exon 2 should be implemented for prediction of clinical benefit from anti-EGFR antibodies in metastatic colorectal cancer.
Worldwide, colorectal cancer (CRC) annually affects one million individuals and causes more than 600 000 deaths [Citation1]. Approximately 20% of the patients have metastatic disease at the time of diagnosis and an additional 20% develop metastases during follow-up [Citation2]. New treatment strategies for metastatic CRC (mCRC) have developed during the last decades resulting in a significant increase in the median overall survival from 6 to 24 months. Standard treatment for mCRC has moved from 5-fluroruracil monotherapy, to combination chemotherapy based on 5-fluorouracil and irinotecan and/or oxaliplatin, and to the more recently introduced biological agents targeted at angiogenesis and epidermal growth factor receptor (EGFR) signalling.
EGF ligand-induced receptor activation elicits its effects via the RAS-RAF-MAPK and PI3K- AKT-mTOR pathways, which promote tumor proliferation, invasion, migration and neovascularization [Citation3]. The EGFR antibodies cetuximab, a chimeric IgG1 antibody, and panitumumab, a humanized IgG2 antibody, both bind to and block the EGFR and have proven effective in all lines of mCRC treatment [Citation4–7]. Mutations in signalling pathways downstream of the EGFR may result in receptor-independent pathway activation that renders the tumors unresponsive to EGFR blockage at the cell surface. Mutations in KRAS codons 12 and 13 (exon 2) are present in 35–45% of CRC and have been established as predictors of lack of response to anti-EGFR treatment [Citation5,Citation8]. Consequently, KRAS exon 2 mutation testing is widely implemented for selection of patients with wild-type mCRC for anti-EGFR treatment (www.emea.europa.eu and www.fda.gov).
Moderate response rates of 40–60% to anti-EGFR treatment, also in KRAS exon 2 wild-type tumors, has motivated assessment of the predictive role of additional alterations, primarily within the RAS-RAF-MAPK and PI3K-AKT-mTOR pathways [Citation9]. Additional mutations in KRAS as well as NRAS have been suggested to infer resistance to anti-EGFR therapies and the European label for panitumumab treatment was recently modified to require testing also for KRAS mutations outside of exon 2 and for NRAS mutations. The added treatment predictive value of mutations in BRAF, PIK3CA and PTEN is more uncertain [Citation2,Citation4,Citation10]. Overall, the added value of extended biomarker analysis has been difficult to determine due to low mutation frequencies for the individual markers. In order to obtain a broader picture and to identify significant genetic predictors, we systematically review the anti-EGFR treatment predictive effect of alterations in KRAS (exons 3 and 4), BRAF, NRAS, PIK3CA and PTEN in mCRC and in a meta-analysis assess the impact of these alterations for overall response rate (ORR), progression-free survival (PFS) and overall survival (OS). These data are the first to report combined outcome results for mutations in KRAS exons 3 and 4 and NRAS.
Material and methods
Literature search
The search strategy was developed in collaboration with a research librarian at the University of Aarhus, Denmark. All published studies including patients with mCRC who had been treated with cetuximab or panitumumab and where data were available on mutations in KRAS and at least one of the genes NRAS, BRAF, PIK3CA or PTEN were eligible for analysis. We constructed and performed searches in Embase and PubMed by combining a disease domain (broadly: “colon cancer” OR “rectum cancer”), a gene domain (broadly: “KRAS” AND [“NRAS” OR “BRAF” OR “PIK3CA” OR “PTEN”]) and a treatment domain (broadly: “panitumumab”OR “cetuximab”) with the Boolean logical “AND”. No constraints related to language or publication type were applied. In PubMed, all search terms were coined as MESH term and as ALL FIELDS (and combined with OR) securing capture of yet un- indexed articles. The combined results of the analog searches in Embase and PubMed were imported into Refworks (RefWorks-COS, ProQuest, UK. http://www.refworks.com/) that allows access and curation of the search result by multiple users with remaining data integrity. Duplicates were identified by a Refworks algorithm and were removed manually after individual scrutiny. Additionally, bibliographies, personal libraries and international gastrointestinal conferences were screened for relevant literature. The final search in both PubMed and Embase was performed on 26 September 2013. Detailed search terms are available from the authors upon request.
Data extraction
All studies identified were independently reviewed by two authors as regards inclusion/exclusion criteria, i.e. mCRC, cetuximab and/or panitumumab treatment, mutation data for KRAS and NRAS, BRAF, PIK3CA or PTEN. Studies where both authors agreed were included/excluded and for studies where the authors disagreed or were uncertain, the decision to include or exclude was reached after joint review. Data extraction was performed in accordance with the guidelines given by The Cochrane Collaboration [Citation11]. All studies included were subjected to data extraction along with a quality assessment using the Newcastle-Ottawa scale for non-randomized studies (NOS, available at http://www.cochrane.org/training/cochrane-handbook). Although this scale is followed by a star-rating system we chose not to use this but rather described the quality of the different studies. Redundant data, which were published in overlapping studies, motivated exclusion. Prior to exclusion authors of the original studies were contacted for clarification. When information on overlapping data was missing, the study with the highest number of patients was included. Data extraction related to study characteristics and design, patient characteristics, treatment, therapy setting, methods for mutation analysis, statistical analysis and outcome results are presented in and .
Table I. Summary of study characteristics.
Table II. Summary of mutation data and outcome measures.
Statistical analysis
The endpoints included were ORR, PFS and OS, corresponding to those used in the studies included. Whenever possible the analyses were based on original raw data. Hazard ratios (HR) and 95% confidence intervals (CI) were extracted. Standard errors corresponding to each HR were re-calculated in agreement to the Cox proportional hazards model. When data on confidence intervals were not available standard errors were calculated from p-values using a Z-test. Cox proportional hazard ratios for PFS and OS were calculated from data and following the procedure given in De Roock et al.'s study [Citation12].
For the meta-analyses, studies with similar clinical and biological features, compliance, biomarker analysis, method of analysis and clinical end-point were pooled. The data was imported into R [R Core Team (2013). R: A language and environment for statistical computing, Vienna, Austria. http://www.R-project.org] using the metabin package by Guido Schwarzer (2013, meta: Meta-Analysis with R. R package version 3.1-1. http://CRAN.R-projects.org/package = meta). All values were inverse variant weighted and pooled HR were calculated using fixed and random effects model. We tested for statistical heterogeneity using the I2 statistics with the hypothesis that the studies were homogenous whenever p > 0.05. Heterogeneity between studies was investigated and subgroup meta-analysis was performed whenever possible to explore the source of heterogeneity. Sensitivity analysis was conducted for all meta-analyses by removing one study at a time to test the robustness of the overall results. To test for presence of publication bias Egger's funnel plots were performed for all analyses.
Results
Literature search and study selection
The literature search identified 366 studies. After exclusion of 43 duplicate studies from the Embase and PubMed, 323 studies published between May 2005 and September 2013 remained for further analysis (Supplementary Figure 1, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.895036). After stepwise exclusion of 140 original studies with contents outside of the scope of our review and of 120 review articles, 63 studies remained for in-depth analysis. A further 24 studies were excluded due to lack of relevant data and 17 studies were further excluded due to overlapping data, failure to separate mutated from wild-type biomarkers or lack of clinical data, which left 22 original studies for inclusion in the meta-analysis ().
Study characteristics
The 22 studies were based on KRAS exon 2 wild-type mCRC treated with anti-EGFR therapies (). The studies were performed in Europe (n = 14), Asia (n = 3), North America (n = 1) or in combinations of these Continents and Africa, South America and Australia (n = 4). The studies were of modest sizes with median 64 patients (range 19–398). ECOG performance status (0–2) was specified in 15 studies. Tumor location was reported in 13 studies with 54–87% colon cancers and 13–46% rectal cancers. Multiple metastatic sites were included with data on liver metastases (present in 21–72% of the patients) reported in seven studies. Of the included studies, seven were based on phase 2 or 3 trials that were performed 1996–2010 [Citation12–19]. All but one of the studies randomized treatment without prior selection based on KRAS mutation testing, whereas one study included part of the patients based on pre-treatment KRAS mutation analysis, which was introduced in 2009 [Citation20]. Treatment consisted of cetuximab in 16 studies, panitumumab in two studies and cetuximab or panitumumab in three studies. Only data from the treatment arm was extracted. Response was evaluated using the RECIST criteria in 21 studies and the WHO criteria in one study. Patients with complete or partial responses were classified as responders in all studies. PFS was in 19 studies defined as the time from treatment initiation to progression or death of any cause, whereas three studies used trial enrolment as entry point in PFS and OS calculations [Citation15,Citation16]. HR for PFS and OS were calculated using the Cox proportional hazard model in all but one study, which used the Mantel-Haenszel method [Citation21]. HRs were adjusted for sex and age in 20 studies and for age only in two study. The majority of studies also adjusted for ECOG performance status and previous lines of treatment.
Biomarker analysis
Paraffin-embedded tumor tissues were used for biomarker analysis in all studies. The fraction of tumors available for testing was reported in seven studies and ranged between 63% and 96%. Among the tumors available for analysis, 74–98% were evaluated for KRAS exon 2 mutations and 74–99% for KRAS mutations in exons 3 and 4, BRAF, NRAS, PIK3CA and alterations in PTEN ().
KRAS mutation testing was performed using sequencing in 14 studies, Pyrosequening in one study and allele-specific PCR in the remaining studies. Mutations in KRAS exon 2 were reported in 24–42%, where studies with mutation rates in the lower range may have applied suboptimal detection methods since KRAS exon 2 mutation rates have been described to increase from about 30% to 50% when alternative mutation detection methods are applied [Citation18]. Mutation screening for KRAS exons 3 and 4, NRAS, BRAF, PIK3CA and PTEN was performed using sequencing in 11 studies, Pyrosequencing in one study and PCR-based methods in six studies, whereas the methods applied were not specified in one study.
Mutations in KRAS exon 3 and 4, NRAS and BRAF are, in general, mutually exclusive with KRAS exon 2 mutations. In contrast, mutations/alterations in PIK3CA (25–60%) and PTEN (50%) may reside together with KRAS exon 2 mutations and were excluded from analysis. Mutation frequencies are provided in .
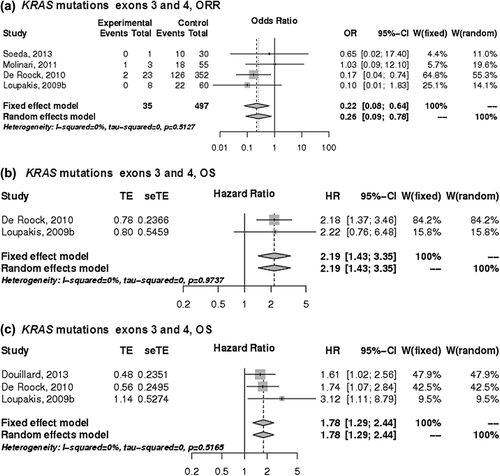
Predictive value of KRAS exons 3 and 4 mutations
The impact on KRAS exons 3 and 4 mutations on ORR to anti-EGFR therapies was evaluated based on five studies [Citation12,Citation14,Citation18,Citation22,Citation23]. KRAS mutations in exons 3 and 4 significantly predicted ORR with an OR of 0.26 (95% CI 0.09–0.78) (). Data on PFS and OS in relation to KRAS exons 3 and 4 mutations allowed for calculations of HR with adjustment for gender, age, cancer center, and previous lines of chemotherapy [Citation12]. Patients whose tumors had KRAS exons 3 and 4 mutations had shorter PFS, HR 2.19 (95% CI 1.43–3.35) and shorter OS, HR 1.78 (95% CI 1.29–2.44) ().
Figure 1. The association between KRAS mutations in exons 3 and 4 with the a) ORR, b) PFS and c) OS in patients with KRAS exon 2 wild-type mCRC treated with anti-EGFR monoclonal antibodies. control, wild-type; events, response; experimental, mutant type; HR, hazard ratio; OR, odds ratio, seTE, standard error of HR; TE, Ln(HR).
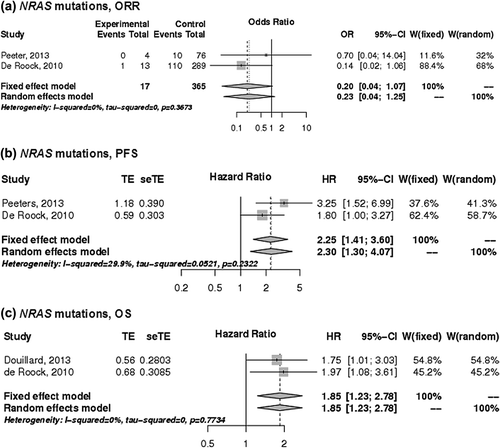
Predictive value of NRAS mutations
Three studies provided predictive information related to mutations in NRAS exons 2, 3 and 4 [Citation12,Citation14,Citation16] and showed a trend towards poor ORR () and a significant adverse effect on PFS with a HR of 2.30 (95% CI 1.30–4.07) and OS with a HR of 1.85 (95% CI 1.23–2.78) ().
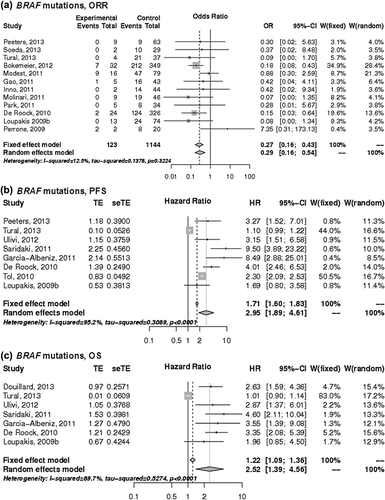
Predictive value of BRAF mutations
Seventeen studies investigated the predictive role of BRAF mutations in KRAS exon 2 wild-type tumors [Citation12–18,Citation20,Citation22–30]. Patients with BRAF mutations had significantly lower ORR compared to patients with BRAF wild-type tumors (17% vs. 45%), which corresponds to an OR of 0.29 (95% CI 0.16–0.54) (). BRAF mutations were also linked to shorter PFS (HR 2.95; 95% CI 1.89–4.61) and OS (HR 2.52; 95% CI 1.39–4.56) compared to BRAF wild-type ().
Figure 3. The associations of BRAF mutations with the a) ORR, b) PFS and c) OS of patients with KRAS wild-type mCRC treated with anti-EGFR monoclonal antibodies. control, wild-type; events, response; experimental, mutant type; HR, hazard ratio; OR, odds ratio, seTE, standard error of HR; TE, Ln(HR).
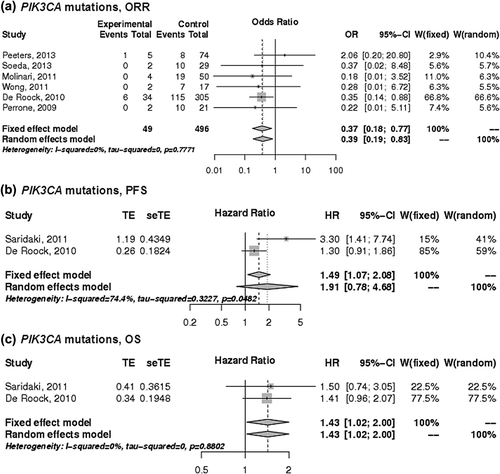
Predictive value of PIK3CA mutations
The predictive value of PIK3CA mutations in exons 9 and 20 was determined from six studies [Citation12,Citation16,Citation18,Citation23,Citation27,Citation28]. PIK3CA mutations significantly predicted poor ORR with an OR of 0.39 (95% CI 0.19–0.83), showed a trend for worse PFS and a significantly shorter OS with a HR of 1.43 (95% CI 1.02–2.00) compared to patients with wild-type tumors (). Data from three studies [Citation12,Citation18,Citation27] allowed for subset analysis related to mutations in PIK3CA exon 9 versus exon 20. Non-significant effects for ORR were identified in both subsets with an OR of 0.61 for PIK3CA exon 9 mutations and OR of 0.24 for exon 20 mutations (Supplementary Figure 2, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.895036).
Figure 4. The associations of PIK3CA mutations with the a) ORR, b) PFS and c) OS of patients with KRAS wild-type mCRC treated with anti-EGFR monoclonal antibodies. control, wild-type; events, response; experimental, mutant type; HR, hazard ratio; OR, odds ratio, seTE, standard error of HR; TE, Ln(HR).
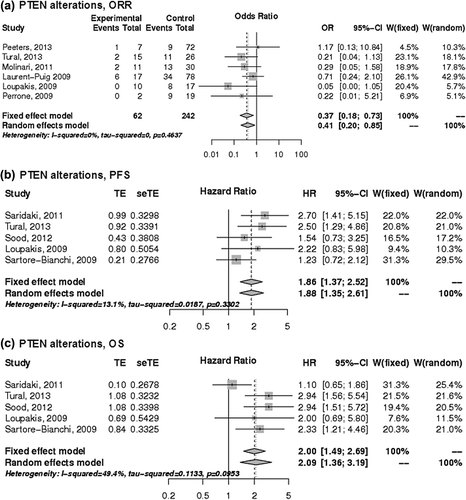
Predictive value of non-functional PTEN
The predictive value of PTEN alterations was assessed in nine studies [Citation16,Citation18,Citation19,Citation21,Citation27–29,Citation31,Citation32] (based on PTEN protein expression in seven studies and PTEN mutations in two studies), whereas data from two studies did not allow for analysis because of missing data on a reported non-significant association between PTEN loss of expression and EGFR-based treatment response [Citation26,Citation32]. Mutation analyses were based on a mix of primary tumors and metastases in four studies, whereas five studies were based on analysis of primary tumor tissues [Citation16,Citation18,Citation28,Citation29,Citation31]. Analysis for ORR related to PTEN mutations could be performed based on 100 patients, nine of whom had mutations and showed a non-significant effect on ORR with an OR of 0.67 (95% CI 0.11–4.15) [Citation16,Citation27]. Tumors with non-functional PTEN (i.e. PTEN mutations and/or reduced PTEN expression) showed significantly lower ORR with an OR of 0.41 (95% CI 0.20–0.85) and shorter PFS (HR 1.88, 95% CI 1.35–2.61) and OS (HR 2.09, 95% CI 1.36–3.19) than patients with functional PTEN (). When the five studies that analyzed PTEN alterations in primary tumors were considered separately, the findings remained significant for ORR and PFS; ORR (OR = 0.46, 95% CI 0.24–0.87), PFS (HR = 2.6, 95% CI 1.64–4.13) and OS (HR = 1.77, 95% CI 0.68–4.64).
Figure 5. The associations of PTEN alterations with the a) ORR, b) PFS and c) OS of patients with KRAS wild-type mCRC treated with anti-EGFR monoclonal antibodies. control, wild-type; events, response; experimental, mutant type; HR, hazard ratio; OR, odds ratio, seTE, standard error of HR; TE, Ln(HR).
The predictive role of multiple biomarker testing
The predictive value of combined biomarker profiles could be evaluated in 16 studies, which consistently demonstrated an improved response prediction when data from more than one marker was considered () [Citation12,Citation15,Citation17–25,Citation27–29,Citation32,Citation33]. The 10 studies that included response data in relation to alterations in KRAS, BRAF, PIK3CA, and PTEN demonstrated response rates that increased from 37% in KRAS exon 2 wild-type tumors to 35–45% when additional KRAS mutations were considered and to 45–54% when alterations in all four genes were considered (). Only one study included NRAS status in the analysis and showed that the response rate increased from 36% in KRAS exon 2 wild-type tumors to 41% in KRAS, NRAS, BRAF, and PIK3CA wild-type tumors [Citation12]. In eight studies, significantly shorter PFS and OS were demonstrated in patients with multiple alterations in KRAS, BRAF, PIK3CA and PTEN [Citation15,Citation17,Citation20,Citation21,Citation23,Citation24,Citation28,Citation29].
Table III. Response rates in metastatic colorectal cancer to anti-EGFR treatment.
Other related analysis
Exploratory analyses for publication bias did not suggest bias for 15 of the 17 analysis performed, whereas BRAF mutation data in relation to PFS and OS were more likely to present positive results (Supplementary Figure 3, to be found online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.895036). Statistical analysis was (because of few included studies) only possible for BRAF and ORR analysis and showed no significant bias (p = 0.51). The studies were heterogeneous, which precluded application of the fixed effect model and motivated use of the random effects model. Sensitivity analyses did, however, not alter the overall conclusions and statistical significance remained also after exclusion of studies with large impact (i.e. large study cohorts and studies with high predictive values).
Discussion
EGFR blockage elicits multiple downstream effects, primarily moderated by the RAS-RAF-MAPK and PI3K-AKT-mTOR signalling pathways. Rational use of targeted therapies requires optimal selection of patients whose tumors are dependent on these pathways. The treatment predictive importance of mutations outside of KRAS exon 2 has gradually been recognized. The studies analyzed herein reveal additional mutations in 4–27% of the tumors (, ) and meta-analysis reveals an independent predictive value from KRAS, NRAS, BRAF, PIK3CA and PTEN ().
Figure 6. The EGFR-related pathways with genes of interest highlighted in gray. The mean mutation frequencies in KRAS exon 2 wild-type primary tumors from the 22 studies included are shown (*refers to mutations found in exons 3 and 4).
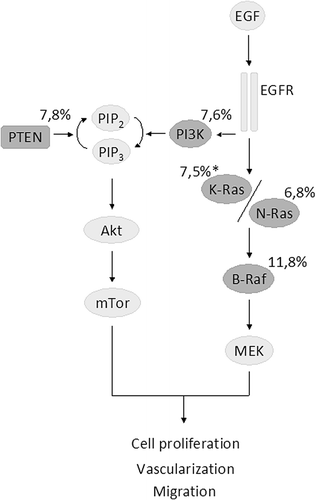
Of the KRAS mutations, 90% are located in exon 2 and 10% in exons 3 and 4 (www.sanger.ac.uk/genetics/CGP/cosmic/). Activating mutations in exons 2 and 3 have been suggested to have similar effects on RAS GTPase activity, whereas exon 4 mutations have been suggested to increase GDP to GTP exchange [Citation10]. The predictive value from extended KRAS mutation testing could be evaluated in five studies and showed reduced response rates to anti-EGFR treatment (). Tumors with KRAS mutations in exons 3 and 4 showed an OR of 0.26, which is comparable to the ORs of 0.13–0.18 reported for KRAS exon 2 mutations [Citation12,Citation24]. The RAS homologue, NRAS, has effector binding domains identical to KRAS. Hence, NRAS mutations in codons 12, 13, 61 and 146 yield effects similar to those from KRAS activation [Citation34]. Meta-analysis showed a non-significant effect on response, but a significantly decreased PFS (HR 2.30) and OS (HR 1.85) for tumors with NRAS mutations (). The consistent effects on response and survival from RAS mutations outside of KRAS exon 2 clearly suggests that mutation testing for anti-EGFR prediction should move from selective KRAS testing to broader RAS testing. Recently, data from the FIRE-3 study were presented in abstract format (not included in our analysis) and lends further evidence in this direction (http://eccamsterdam2013.ecco-org.eu/Scientific-Programme/Abstract-search.aspx? abstractid = 8953). The FIRE-3 study compares FOLFIRI plus cetuximab to FOLFIRI plus bevacizumab head to head in a first-line setting. Other RAS mutations (KRAS exon 2/3/4 and NRAS exon 2/3/4) were detected in 14% and BRAF (V600E) mutations in 11%. The response rate in the FOLFIRI/cetuximab arm showed an increased ORR for patients (N = 301) with RAS wild-type tumors compared to 55 patients with RAS mutations (76.0% vs. 42.9%). The FIRE-3 study also suggests an effect on OS, which reached 33.1 months in RAS wild-type patients compared to 19.1 months for RAS mutated patients treated with cetuximab.
BRAF mutations seem to have dual roles with well-established adverse prognostic effects [Citation5] and a suggested treatment predictive effect in our meta-analysis with an OR for ORR of 0.29, and HRs for PFS of 2.95 and for OS of 2.52 (). The BRAF mutation rates in KRAS wild-type tumors ranged from 4% to 21%, which may reflect the patient population studied, as the mutation confers poor prognosis, BRAF mutation subgroups decline in later lines of therapy. Clinical application of a negative treatment predictor in a prognostically unfavorable subset is challenging. Patients with BRAF mutated tumors have a shorter PFS and OS, irrespective of treatment [Citation13,Citation14,Citation16], but additionally demonstrate a low chance for response (ORR = 0–8%) to monoclonal EGFR antibodies even in later lines of therapy [Citation12,Citation16]. We believe that combination chemotherapy, potentially combined with bevazicumab, should remain standard in asymptomatic patients with BRAF mutated mCRC, whereas patients with symptoms or in need of down-staging could also be candidates for triple combination chemotherapy and evaluation of novel treatment principles.
PIK3CA mutations occur in 12–15% of mCRC. Prognostic and treatment predictive effects have not been firmly established though promising observations have linked use of aspirin to a favorable prognosis in patients with PIK3CA mutated tumors [Citation35]. This link is likely inferred by aspirin-induced inhibition of cyclooxygenase 2 that in turn down-regulates phosphatidylinositol 3-kinase (PI3K) signaling. Our analysis reveals a significant influence from PIK3CA mutations as regards ORR with an OR of 0.39 and OS with a HR of 1.43 (). Hence, suggested predictive effects related to aspirin as well as anti-EGFR therapies suggest that PIK3CA mutation analysis should be implemented in CRC.
PTEN alterations, i.e. combined analysis of the 7–9% of tumors with PTEN mutations and the 19–58% of tumors with loss of PTEN protein expression, predicted poor ORR with an OR of 0.41 for response and reduced clinical benefit from anti-EGFR treatment with a HR for PFS of 1.88 and a HR for OS of 2.09 (). These findings strongly encourage further prospective evaluation of the predictive value of PTEN. Though immunohistochemistry needs further validation as regards its sensitivity and specificity as a marker for PTEN inactivation, it may be a preferred method of analysis since PTEN is silenced through various mechanisms, e.g. mutation, loss of heterozygosity, and promoter methylation. Studies of the anti-EGFR treatment predictive value of PTEN and the usefulness of primary tumor tissue versus metastatic tissue have reached different conclusions, which may relate to analytical differences [Citation27,Citation31,Citation32,Citation36]. Our data suggest that PTEN status for anti-EGFR prediction may be evaluated in primary tumors and well as based on materials that combine primary tumors and metastases.
A recent review by Yang et al. identified significant predictive information related to anti-EGFR treatment from mutations in BRAF and PIK3CA and non-functional PTEN, whereas data on the predictive value of mutations in KRAS exons 3 and 4 and NRAS were not included [Citation37]. Regarding BRAF, 3–4 of the eight studies we used to determine the effect on PFS and OS were also analyzed by Yang et al. As expected, the meta-analyses reached comparable HRs, i.e. HRs for PFS of 2.67 in our study and 2.59 in Yang et al. and HR for OS of 2.37 by us and 2.74 by Yang et al. For PIK3CA mutations and PTEN alterations, 2/2 and 4/5 studies were overlapping and the results thus identical for PIK3CA and similar for PTEN with HRs for PFS of 1.88 by us and 1.75 by Yang et al. and HRs for OS of 2.09 and 1.85, respectively.
The predictive information from combined biomarker panels that consider mutations/alterations in KRAS, BRAF, PI3KCA and PTEN was evaluated in 16 of the studies included in our review. Combined analysis of KRAS and BRAF mutations increased response rates marginally, from 38% to 39%, which may be linked to the poor prognosis in BRAF mutated tumors () [Citation12,Citation18,Citation24,Citation27,Citation33]. The combined effect from KRAS, BRAF and PIK3CA mutations was evaluated in four studies and increased response rates from 38% to 42%, whereas and quadruple wild-type tumors (for KRAS, BRAF, PIK3CA and PTEN) showed response rates of 51% () [Citation18,Citation20,Citation23,Citation27].
In summary, the independent predictive values from KRAS, NRAS, BRAF, PIK3CA and PTEN demonstrated in our meta-analysis and the increased response rates in tumors that remain wild-type after multiple-biomarker assessment, strongly suggest treatment predictive testing in mCRC should apply biomarker panels in order to optimize identification of patients who will benefit from anti-EGFR treatment.
Supplementary Figures 1–3
Download PDF (502.9 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30.
- Recommendations from the EGAPP Working Group: Can testing of tumor tissue for mutations in EGFR pathway downstream effector genes in patients with metastatic colorectal cancer improve health outcomes by guiding decisions regarding anti-EGFR therapy?. Genet Med 2013;15:517–27.
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008;358:1160–74.
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: The PRIME study. J Clin Oncol 2010;28: 4697–705.
- Van Cutsem E, Kohne CH, Lang I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011–9.
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second- line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706–13.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337–45.
- Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091–6.
- Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: A systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9: 962–72.
- Janakiraman M, Vakiani E, Zeng Z, Pratilas CA, Taylor BS, Chitale D, et al. Genomic and biological characterization of exon 4 KRAS mutations in human cancer. Cancer Res 2010;70:5901–11.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration 2009; www.cochrane-handbook.org.
- De Roock W, Claes B, Bernasconi D, De SJ, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol 2010;11:753–62.
- Bokemeyer C, Van CE, Rougier P, Ciardiello F, Heeger S, Schlichting M, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48: 1466–75.
- Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34.
- Modest DP, Jung A, Moosmann N, Laubender RP, Giessen C, Schulz C, et al. The influence of KRAS and BRAF mutations on the efficacy of cetuximab-based first-line therapy of metastatic colorectal cancer: An analysis of the AIO KRK-0104-trial. Int J Cancer 2012;131: 980–6.
- Peeters M, Oliner KS, Parker A, Siena S, Van CE, Huang J, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res 2013;19:1902–12.
- Tol J, Dijkstra JR, Klomp M, Teerenstra S, Dommerholt M, Vink-Borger ME, et al. Markers for EGFR pathway activation as predictor of outcome in metastatic colorectal cancer patients treated with or without cetuximab. Eur J Cancer 2010;46:1997–2009.
- Molinari F, Felicioni L, Buscarino M, De DS, Buttitta F, Malatesta S, et al. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res 2011;17:4901–14.
- Sartore-Bianchi A, Di NF, Nichelatti M, Molinari F, De DS, Saletti P, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PLoS One 2009;4:e7287.
- Ulivi P, Capelli L, Valgiusti M, Zoli W, Scarpi E, Chiadini E, et al. Predictive role of multiple gene alterations in response to cetuximab in metastatic colorectal cancer: A single center study. J Transl Med 2012;10:87.
- Sood A, McClain D, Maitra R, Basu-Mallick A, Seetharam R, Kaubisch A, et al. PTEN gene expression and mutations in the PIK3CA gene as predictors of clinical benefit to anti-epidermal growth factor receptor antibody therapy in patients with KRAS wild-type metastatic colorectal cancer. Clin Colorectal Cancer 2012;11:143–50.
- Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 2009;101:715–21.
- Soeda H, Shimodaira H, Watanabe M, Suzuki T, Gamoh M, Mori T, et al. Clinical usefulness of KRAS, BRAF, and PIK3CA mutations as predictive markers of cetuximab efficacy in irinotecan- and oxaliplatin-refractory Japanese patients with metastatic colorectal cancer. Int J Clin Oncol 2013;18:670–7.
- Gao J, Wang TT, Yu JW, Li YY, Shen L. Wild-type KRAS and BRAF could predict response to cetuximab in Chinese colorectal cancer patients. Chin J Cancer Res 2011;23:271–5.
- Inno A, Di SM, Cenci T, Martini M, Orlandi A, Strippoli A, et al. Is there a role for IGF1R and c-MET pathways in resistance to cetuximab in metastatic colorectal cancer?Clin Colorectal Cancer 2011;10:325–32.
- Park JH, Han SW, Oh DY, Im SA, Jeong SY, Park KJ, et al. Analysis of KRAS, BRAF, PTEN, IGF1R, EGFR intron 1 CA status in both primary tumors and paired metastases in determining benefit from cetuximab therapy in colon cancer. Cancer Chemother Pharmacol 2011;68: 1045–55.
- Perrone F, Lampis A, Orsenigo M, Di BM, Gevorgyan A, Losa M, et al. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol 2009;20:84–90.
- Saridaki Z, Tzardi M, Papadaki C, Sfakianaki M, Pega F, Kalikaki A, et al. Impact of KRAS, BRAF, PIK3CA mutations, PTEN, AREG, EREG expression and skin rash in >/ = 2 line cetuximab-based therapy of colorectal cancer patients. PLoS One 2011;6:e15980.
- Tural D, Batur S, Erdamar S, Akar E, Kepil N, Mandel NM, et al. Analysis of PTEN, BRAF and PI3K status for determination of benefit from cetuximab therapy in metastatic colorectal cancer patients refractory to chemotherapy with wild-type KRAS. Tumour Biol 2014; 35:1041–9.
- Garcia-Albeniz X, Pericay C, Alonso-Espinaco V, Alonso V, Escudero P, Fernandez-Martos C, et al. Serum matrilysin correlates with poor survival independently of KRAS and BRAF status in refractory advanced colorectal cancer patients treated with irinotecan plus cetuximab. Tumour Biol 2011;32:417–24.
- Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, et al. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol 2009; 27:5924–30.
- Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, et al. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol 2009;27:2622–9.
- Wong NS, Fernando NH, Nixon AB, Cushman S, Aklilu M, Bendell JC, et al. A phase II study of capecitabine, oxaliplatin, bevacizumab and cetuximab in the treatment of metastatic colorectal cancer. Anticancer Res 2011;31: 255–61.
- Hancock JF. Ras proteins: Different signals from different locations. Nat Rev Mol Cell Biol 2003;4:373–84.
- Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med 2012;367: 1596–606.
- Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 2007;97:1139–45.
- Yang ZY, Wu XY, Huang YF, Di MY, Zheng DY, Chen JZ, et al. Promising biomarkers for predicting the outcomes of patients with KRAS wild-type metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: A systematic review with meta-analysis. Int J Cancer 2013;133:1914–25.