Abstract
Background. Intraoperative radiotherapy (IORT) has been given for primary and locally recurrent rectal cancer for 30 years. Still, its effect is not clear.
Material and methods. PubMed and EMBASE search for papers after 1989 on surgical treatment and external beam radiotherapy (EBRT) for primary advanced and locally recurrent rectal cancer, with and without IORT. From each center the most recent paper was generally selected. Survival and local recurrence at five years was tabulated for the total groups and separate R-stages. Also, the technique for IORT, use of EBRT and chemotherapy as well as surgical approach was registered.
Results. In primary cancer 18 papers from 14 centers were tabulated, including one randomized and five internally comparing studies, as well as seven studies without IORT. In locally recurrent cancer 18 papers from 13 centers were tabulated, including four internally comparing studies and also five without IORT. Overall survival (OS) and local recurrence rate (LRR) were higher for primary cancer compared to recurrent cancer. Patients with R0 resections had better outcome than patients with R1 or R2 resections. For primary cancer OS and LR rate of the total groups and R0 stages was not influenced by IORT. An effect on R1/R2 stages cannot be excluded. The only randomized study (primary cancer) did not show any effect of IORT.
Conclusion. IORT does not convincingly improve OS and LR rate for primary and locally recurrent rectal cancer. If there is an effect of IORT, it is small and cannot be shown outside randomized studies analyzing the separate R stages.
The frequency of local recurrence (LR) has been extensively reduced after the introduction of total mesorectal excision (TME) surgery [Citation1,Citation2] for rectal cancer. However, still about 10% of the patients experience a local recurrence. For locally advanced primary cancer LR rate may be higher [Citation3]. Many of these patients have LR only and no distant metastases at the time of death, and might therefore possibly be cured if the recurrence could be adequately treated [Citation4].
Preoperative external beam radiotherapy (EBRT), in recent years often combined with sensitizing chemotherapy, has reduced the frequency of local recurrences in locally advanced rectal cancer [Citation5].
Intraoperative radiotherapy (IORT) in combination with EBRT was introduced at the Massachusetts General Hospital and the Mayo Clinic for the treatment of locally advanced and recurrent rectal cancer. Compared to historical controls, patients having IORT were reported to have higher overall survival (OS) and lower LR rate [Citation6,Citation7]. These results were supported by some other studies [Citation8–11], although more recent studies have shown conflicting data [Citation12–15]. Randomized studies have been requested since 1986 but are still virtually lacking [Citation16]. Only one recent study is available on primary rectal cancer combined with external radiotherapy (EBRT) [Citation17] and one without EBRT [Citation18]. These studies showed no effect of IORT on survival or LR rate.
Using IORT the radiosensitive bowel and bladder can be excluded from the radiation field. A higher dose to the tumor bed can therefore be applied. It has been suggested that a single large dose of radiation is equivalent to 2–3 times the same dose when given as 2 Gy fractions [Citation19]. Others have argued that by applying only 1 fraction of radiotherapy a number of the tumor cells will be in a relatively radioresistant state (G0), and therefore will not be susceptible to therapy.
IORT can be given by three different techniques: Electrons (IOERT), high dose rate (HDR) brachytherapy and low dose rate (LDR) brachytherapy using iodine 125 seeds.
IOERT is most often delivered by means of an accelerator during a few minutes. The energy of electrons can be varied according to the depth of tissue to be treated. The radiation dose applied is usually enhanced with increasing tumor burden. Thus, patients with R0- resections are typically given 10–12 Gy, R1-resections 12–15 Gy and R2- or non-resections 15–20 Gy. The radiation is delivered through a cone, usually towards the tumor bed. Some centers routinely irradiate the presacral area where most LR appear [Citation20,Citation21]. A large tumor bed can be difficult to adequately cover with IOERT. Therefore, a few departments give multiple fields, with a risk of overlapping and consequently overdosing problems.
HDR radiotherapy treatment is delivered through parallel catheters in a flexible plastic flap. The size of the flap is adjusted according to the region at risk and is packed onto the curving pelvic surface. The packing will displace the normal organs which thereby avoid radiation damage. It is a disadvantage that the radiation procedure takes nearly an hour, and the penetration depth is only 0.5–1 cm.
Irradiation with iodine 125 seeds is presently not very much applied, and is therefore not further discussed.
In USA and Europe IORT is mostly combined with EBRT, as the dose of a single intraoperative fraction is considered too low [Citation22].
In primary rectal cancer IORT has been given for “locally advanced cases” which may have included T3/T3 +/T4 or N+ cases, i.e. both “intermediary risk” (IR) and high-risk true “locally advanced” (LA) cancers [Citation3] in varying mixtures.
A varying number of patients with locally recurrent rectal cancer (LRRC) have been treated with EBRT for their primary cancer. These patients may benefit from a reduced dose of preoperative reirradiation [Citation22].
Chemotherapy has often been combined with radiotherapy. Various chemotherapy regimens have been used, according to changing standards.
Around 40 reviews on IORT for rectal cancer have been published. All of them claim that IORT has an effect. Three recent reviews have concluded that IORT reduces the frequency of LR [Citation23–25] while a slightly older review claims improvement of both disease-free survival (DFS) and LR rate [Citation26].
Studies on IORT have now been published for nearly 30 years. Still the effect of IORT in rectal cancer treatment is not clear. Here we give a review of the available literature.
Material and methods
Data
Studies of IORT in rectal cancer published after 1989 were collected by searching PubMed and EMBASE and our own database, for studies published in English before 1 June 2013. The keywords IORT, IOERT, EBRT, surgery and locally advanced primary or locally recurrent rectal cancer were used.
Non-IORT studies were preferably selected from randomized studies with chemoradiotherapy including 5FU or capecitabine on locally advanced rectal cancer with the Keywords: EBRT, surgery and locally advanced primary or locally recurrent rectal cancer.
We recorded the preoperative cancer stage and imaging, level of evidence, the preoperative radio- and chemotherapy as well as five-year survival and LR rate.
Papers were excluded when: 1) patients with primary and locally recurrent rectal cancer were analyzed and reported together; 2) IORT was used without EBRT; and 3) actuarial five-year survival or local recurrence rate could not be stipulated. The most recent paper, including the largest number of patients, was picked among several publications from the same department, unless different patient populations were studied. Studies including surgery only were also excluded as preoperative EBRT is considered better than surgery only in LARC, although this is challenged in a recent study from the Mayo Clinic [Citation27].
Unfortunately, most of the available studies on IORT are not suitable for meta-analysis. In a recent review of 29 studies including 3003 patients only five studies on primary cancer and two studies on locally recurrent cancer, including a total of 631 patients, could be used for meta-analysis [Citation25]. These studies were small as only two included more than 50 patients in each arm. The rest of 2372 operated patients were of no value for the evaluation of the effect of IORT.
We have here selected well accepted, large studies with optimal non-IORT treatment which means preoperative irradiation with or without sensitizing 5FU or capecitabine-based chemotherapy.
The available studies are divided into four groups:
Randomized studies (level of evidence II) [Citation3];
Studies comparing an IORT group with a “control group” (level of evidence III);
IORT studies without “control group” (level of evidence V);
Contemporary studies without IORT (level of evidence V).
Statistical analysis
As a meta-analysis could not be performed we give in this review the range and mean values for survival and local recurrence rate presented in the different publications. The heterogeneity in design and reporting of the IORT studies implies that weighted means should not be used. We anticipated that a definite effect of IORT would influence the mean values of survival and local recurrence rate compared to those of patients not receiving IORT. Moreover, the results of individual studies with and without IORT should ideally not overlap.
For the comparing studies the IORT and non-IORT results were included in the respective groups.
Results
Primary cancer
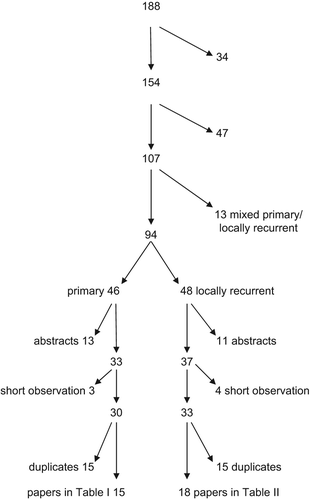
Totally, we read 188 abstracts on IORT. Fifteen of 46 relevant papers were selected for this review (flow chart in ). IORT treatment was performed in 12 centers.
Figure 1. Flow chart showing reason for excluding references from PubMed, EMBASE and own database from inclusion in and .
Data from the 15 individual studies are shown in . Totally 1929 patients were given IORT while 2343 were in the non-IORT groups. The comparing studies include considerably fewer patients than the non-comparing ones. also shows that there were slightly more locally advanced cases in the non-IORT/non-comparing group while there were similar numbers of intermediary risk and locally advanced cases in the other three groups. It should be noticed that only a few studies routinely applied MRI for the preoperative diagnosis.
The only randomized study was stopped prematurely. This study is relatively small and therefore considered to be at level of evidence II. Other studies were mostly prospectively recorded consecutive series in which IORT was given on special indications. Some of the non-IORT studies were investigating other factors. These were considered level of evidence V.
The frequency of R0 resections varied from 59% [Citation28] to above 90% [Citation5,Citation29–32]. However, the frequency of R-stages in different studies could not always be compared and was not always reported [Citation11,Citation17,Citation28,Citation33–38]. Thus the results must be interpreted with caution.
Table I. Studies with IORT on primary rectal cancer compared with non-IORT studies. Author with reference number in square brackets. Observation time (Obs) is given in months. Percentages are given for the following parameters: risk stage, surgical R stage, patients given intraoperative radiotherapy (IORT), external radiotherapy (EBRT), and chemotherapy (ChT). The effect of IORT is given as percent of overall survival (OS), cancer specific survival (CSS) or disease-free survival (DFS) and local recurrence (LR) percentage for the total group and R stages.
Treatment with IORT was heterogenic. Some centers used HDR-IORT [Citation13,Citation14,Citation39] while the majority applied IOERT. Doses were mostly 10–15 Gy depending on amount of residual tumor, while some routinely used 10 Gy [Citation11,Citation14].
Indications for giving IORT varied from treatment to all cases [Citation38] to treatment of only micro- or macroscopic remaining cancer or in cases with a free resection margin less than 1 mm [Citation32], 2 mm [Citation14] or 5mm [Citation40]. Others have not specified the indication for IORT treatment. Some have omitted IORT due to lack of availability of IORT [Citation12]. The frequency of IORT given ranged from 9% [Citation32] to 100% (most studies). Most departments directed IORT towards the tumor bed, some routinely towards the presacral space [Citation31,Citation38].
When IORT was not given to all patients, IORT and non-IORT groups could sometimes be analyzed separately [Citation11,Citation12,Citation14,Citation26,Citation37,Citation41,Citation42].
EBRT also varied to some extent. Most centers gave preoperatively approximately 50 Gy in 1.8–2 Gy fractions, both in the IORT and non-IORT groups, varying from 20 Gy [Citation33] to 57 Gy [Citation31].
Use of sensitizing chemotherapy varied from 0% [Citation14,Citation43] to almost 100% (most studies). Many centers gave adjuvant postoperative chemotherapy varying from 25% [Citation41] to 99% [Citation29].
Survival and LR rate
The mean and limits for survival and LR rate are shown for the IORT and non-IORT studies in . There is no indication of improved survival for patients treated with IORT, neither in the total group, nor in the R0 group, compared to corresponding non-IORT groups. Few R1 and R2 patients are reported. LR rate is slightly higher in the total IORT group, but is slightly lower for patients with R0 resections. For the R1-2 groups a higher LR rate was found in the non-IORT group. It is clear that patients with R0 resections do better for both OS and LR rate compared to those with R1-2 resections.
Table II. Primary cancer. Limits, mean and number of studies giving overall survival and local recurrence for the total group of patients and the separate R stages.
The most frequent conclusion of the studies is that achievement of R0 resection is more important than giving IORT [Citation32,Citation34,Citation40,Citation44]. It is also concluded that IORT should be given towards the tumor bed and not routinely towards the presacral area [Citation45]. One of the studies suggested that IORT reduces LR by interaction with subsequent adjuvant chemotherapy [Citation34].
Among the papers with no internal comparison one reported reduction of infield recurrences, although not in R1 resections [Citation32]. Another suggested that IORT reduced infield recurrences by 20–30% [Citation31], whereas one found that a substantial number of presacral recurrences appeared in spite of IORT [Citation45].
The median or mean observation time varied from 25 [Citation14] to 74 months [Citation37], but a number of studies did not give this information.
There is only randomized study [Citation17] among the comparing studies. This study had the highest number of patients included. However, no effect on survival or LR rate was found.
In a small study [Citation14] comparing 11 IORT patients and eight non-IORT patients, improved survival and reduced LR rate were found for patients with R1-2 resections.
A study on 29 patients with R0 resections of locally advanced cancers found low LR rate (0%) after IORT [Citation46]. This is definitely better than the LR rate reported from the same institution on less advanced intermediary risk cancers similarly treated [Citation11].
Locally recurrent cancer
Eighteen papers from 13 centers were selected from originally 48 papers (, ), including 1174 patients. Also here the comparing studies included fewer patients than the non-comparing ones. In five papers 354 patients with locally recurrent cancer not given IORT, were reported.
Table III. Studies with IORT on locally recurrent rectal cancer compared with non-IORT studies. Author with reference number in square brackets. Observation time (Obs) is given in months. Percentages are given for the following parameters: surgical R stage achieved, patients given intraoperative (IORT), external radiotherapy (EBRT), and chemotherapy (ChT). The effect of IORT is given as overall survival (OS), cancer specific survival (CSS), disease free survival (DFS) and local recurrence (LR) percentage for the total group and R stages.
The frequency of R0 resections varied from 0% [Citation47,Citation48] to 79% [Citation42,Citation49], being 37% in the largest study on 607 patients from the Mayo Clinic, also including some colon cancers [Citation22]. The frequency of R-stages was not always reported [Citation8,Citation44]. The IORT schedule and indications were heterogenic also in recurrent rectal cancer. The fraction given IORT varied from 18% [Citation50] to 100%. One center used HDR-IORT [Citation51] while the majority applied IOERT. Dosage was nearly always 10–20 Gy, depending on amount of tumor tissue left, while it varied from 7.5 Gy to 30 Gy for previously irradiated patients [Citation22]. Most departments directed IORT towards the tumor bed, a few routinely gave IORT to the presacral area [Citation8,Citation21].
The indications for IORT varied, in some studies based upon the preoperative examinations [Citation52], and in others based upon perioperative findings, with treatment of only micro- or macroscopic remaining cancer [Citation47], or in cases with a free resection margin less than 1 mm [Citation53] or 10 mm [Citation54]. When IORT was not given to all patients, results in IORT and non-IORT groups could sometimes be reported separately [Citation10,Citation12,Citation42,Citation47].
EBRT also varied from one study to another, although most centers gave preoperatively approximately 50 Gy. In recent studies of IORT in recurrent rectal cancer, large fractions (up to 66%) of patients had previously received EBRT in the primary situation [Citation13,Citation22,Citation28,Citation48,Citation51,Citation55]. In such patients the EBRT dose varied from 0 to 74 Gy [Citation13,Citation22,Citation28, Citation44,Citation47,Citation48,Citation51,Citation55].
Use of sensitizing chemotherapy differed between studies, varying from 0% [Citation8,Citation10,Citation12] to 100% [Citation56] of the patients reported. Some centers gave adjuvant postoperative chemotherapy, varying from 25% [Citation41] to 100% [Citation57]. The chemotherapy regimen used was not reported in some studies [Citation42,Citation47,Citation57–59].
The lack of a generally accepted staging system for recurrent rectal cancer makes it impossible to correctly compare the groups selected for operation. The local recurrences obviously ranged from smaller anastomotic recurrences [Citation8,Citation21], to large infiltrating tumors needing extensive surgery like sacrectomies [Citation10,Citation49,Citation50].
The median or mean observation time varied from 15 to 63 months, but was not given in some studies [Citation12,Citation42,Citation57].
Survival and LR rate
OS and LR rate are shown for the IORT and non-IORT studies in . IORT does not seem to influence either the OS or LR rate. Unfortunately, few studies specify the results for R1 and R2 resections, especially in the non-IORT group.
Table IV. Locally recurrent cancer. Limits, mean and number of studies giving overall survival and local rerecurrence for the total group of patients and the separate R stages.
The authors frequently stated that it is more important for the outcome to obtain an R0 resection than to give an IORT boost [Citation8,Citation12,Citation15,Citation21,Citation22,Citation44, Citation51,Citation54,Citation60].
In a study of patients with mostly R2 resections [Citation47] IORT was found to statistically improve both OS and LR rate. One study [Citation42] found improved survival for nine patients with R0-R1 resections with IORT compared to eight patients without IORT. Another study found better OS and LR rate at three years after IORT compared to historical controls [Citation10]. However, they performed more aggressive surgery and had twice as many R0 resections in the IORT group. The only comparing study which did not report any effect of IORT [Citation12] included few patients in each R-stage.
Discussion
In this overview of IORT for locally advanced and locally recurrent rectal cancer, we have included reports from altogether 36 studies of various type and quality. As we do not have results from large randomized trials it is difficult to draw definite conclusions regarding the question: Does IORT work at all? However, by giving the summarized results as mean with variations, comparisons between groups may give important indications of effects on outcome.
By calculating the mean and variation of results from available studies we have found clear differences in survival and local failure between primary cancers and locally recurrent cases, as well as for R0 resections versus R1-2 resections. This is according to previous reports, and shows that this approach may be useful to investigate differences in outcome between groups.
Our main conclusion is that no definite improvement by IORT has been documented. In primary cancer, there was neither any effect on OS nor LR rate for patients with R0 resections or the total group (including R0, R1 and R2 resections). For R1 and R2 patients no conclusion regarding OS could be made. The mixed R1-2 results possibly suggested an effect of IORT on LR rate. For locally recurrent cancer there was no effect of IORT on OS, neither for the total group, nor for the separate R-stages. This also applies to the LR rates.
The results clearly show the importance of separately analyzing and reporting primary and locally recurrent cancers and their specific R-stages.
Are the IORT and non-IORT studies comparable? Using the TNM staging system studies in primary cancer should be relatively comparable with regard to extent of cancer. However, magnetic resonance imaging (MRI) or computed tomography (CT) was in many of the studies not routinely used and the preoperative diagnosis therefore uncertain [Citation3]. In locally recurrent cases, no generally accepted staging system exists. Therefore, comparisons of groups of patients are even more uncertain than in primary cases. Differences in selection of patients and quality of surgery can profoundly influence the results in such cases. For some of these studies good results might be partly explained by a high frequency of anastomotic recurrences [Citation8,Citation21].
The reports on primary cancer include patients with intermediary risk and locally advanced cancers. However, preoperative examinations suggested less locally advanced cancers in the IORT/non-comparing group. In contrast, the IORT comparing group included patients with a higher R-stage. This was supposed to be the reason why there was hardly any LR in the non-IORT R0 group of two studies. If there was any effect of IORT this could only be small as the presence of LR in R1 resected cases shows that IORT cannot eradicate even microscopically remaining cancer [Citation21,Citation43]. Treatment with IORT might have reduced the inter-study variation in results. For IORT studies this was similar to that of the non-IORT studies. A small effect could be hidden by the heterogeneity in technique (HDR/IOERT), dosage, localization [Citation20,Citation31,Citation34], definition of “close” margin [Citation14,Citation32,Citation40], differences in frequency of IORT given [Citation13,Citation28,Citation41] and inclusion of varying numbers of previously irradiated patients in the studies of locally recurrent cases.
The inclusion of M1 stages in some reports [Citation21,Citation42,Citation49,Citation60,Citation61] confounds the evaluation of IORT effect even more.
In primary cancers patients with R0 resections will have a low rate of local recurrences and IORT effect can therefore be difficult to identify. Our results are in line with this and strongly support the previous suggestion that IORT should not routinely be given to R0 resections of cancers with intermediary risk [Citation17,Citation25]. In R1 resections with microscopic remaining cancer the LR frequency will be higher and this should therefore be the ideal group for the identification of a potential IORT effect. Also patients with R0 resections of locally recurrent cancer with a relatively high rate of local rerecurrences, could be expected to improve with IORT treatment. Similarly, one would expect that cases with R1 resections of local recurrences could be susceptible to IORT. It is therefore unfortunate that the R-stage is not specified in several of the studies of both the IORT and non-IORT groups of primary cancer as well as local recurrent cancer [Citation11,Citation35–37,Citation43,Citation56,Citation59,Citation62,Citation63]. As locally recurrent rectal cancer grow in less well-oxygenated scar tissue, and theoretically can be radioresistant in cases with previous irradiation, one might imagine that all cases with locally recurrent cases would need higher doses of IORT. This was not discussed in any study. The fact that the Mayo Clinic in their large studies consistently reports better results than most other centers for R2 resected LRRC could be due to their use of 20 Gy IORT [Citation22], which is higher than used by most others.
The R-staging can be difficult [Citation64] and therefore inaccurate, and is particularly problematic in locally recurrent rectal cancer. This can explain part of the variation of results within separate R-stages and also the higher frequency of LR in R0 stage of LRRC compared to primary cancer. In fact, none of the studies declare the number of microscopic slides examined for the R-staging.
The most frequent conclusion reported is that IORT is not able to improve the results after a R1 resection to the level obtained after a R0 resection. This applies for both primary [Citation32,Citation40,Citation44,Citation46] and recurrent cancer [Citation8,Citation12,Citation15,Citation22,Citation51,Citation54,Citation60]. However, conclusions in various studies are contradictory [Citation8,Citation13].
A reappraisal of the conclusions of comparing studies showing statistical significant improvement with IORT boost suggests that these results must be interpreted with caution.
Among the studies on primary cancers the only randomized study [Citation17] neither found effect on survival nor on LR rate. This might be due to a low number of LR. This conclusion is in contrast to the conclusion made by other authors, based on consecutive, similar patient groups [Citation31,Citation33,Citation34,Citation38].
All other studies included low number of patients where differences in tumor biology or inaccurate staging might profoundly influence the results. One study included less than 10 patients in each comparing group [Citation42], two others less than 20 [Citation10,Citation14] and a fourth less than 30 patients in one of the groups compared [Citation26]. Also one of the studies had surprisingly low estimated three- (11%) and five-year OS (0%) in the historic control non-IORT group [Citation10] and twice as many R0 resection in the aggressively operated IORT group. In fact this study is often cited as proving the effect of IORT [Citation65]. However, one of the studies reported no LR after IORT [Citation26] which is better than for patients with less extensive tumors reported from the same department [Citation11]. The results also seem inconsistent as one study on locally advanced cancers found improvement in LR rate after R0 resection [Citation26], while another in contrast found improved OS and LR rate after R1-2 resections [Citation14]. For locally recurrent cases one study found effect on OS of the total group [Citation10], another on OS of R0-1 resections [Citation42] and a third found improvement of both OS and LR after R2 resections [Citation47].
What is the biological effectiveness of radiation therapy given as IORT? Interestingly, the recent paper from the Mayo Clinic [Citation22] suggested that the additive effect of EBRT and IORT may be lower than previously estimated [Citation6]. It was suggested that the combined dose of EBRT 50 Gy plus intraoperative irradiation 12 Gy given four weeks later, might be equivalent to 66 Gy in 2 Gy fractions, and that EBRT reirradiation of 30 Gy plus 12 Gy IORT immediately thereafter for previously irradiated patients is equivalent to 63 Gy (Haddock pers. comm.).
Conclusion
The studies here referred, including both IORT and non-IORT-treated patients, suffer from a lack of standardization in the presentation of the results, making the groups difficult to compare. The results reported for the IORT and non-IORT groups, both for primary and recurrent cancer and their R-stages, are difficult to interpret. The wide variation in results for IORT-treated patients is within the same limits as for the non-IORT-treated patients. Also the partly contradicting conclusions of the IORT papers do not provide any convincing evidence of an IORT effect.
Interestingly, a new model for estimation of the combined effect of EBRT and IORT has suggested less additive effect than previously assumed and therefore theoretically a possibly smaller benefit from IORT may be expected.
After 30 years of treatment with IORT it is clear that IORT unfortunately has not had a great impact on treatment results. It still cannot be completely excluded though, that there might be an effect on LR rate.
Only large randomized studies can establish whether IORT is of any therapeutical value. As far as we know, no such study is ongoing or planned. Is IORT then of clinical relevance?
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1: 1479–82.
- Wibe A, Moller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, et al. A national strategic change in treatment policy for rectal cancer – implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002;45:857–66.
- Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24(Suppl 6):vi81–vi88.
- Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 1974;34:1278–92.
- Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351: 1731–40.
- Gunderson LL, Nelson H, Martenson JA, Cha S, Haddock M, Devine R, et al. Locally advanced primary colorectal cancer: Intraoperative electron and external beam irradiation +/2 5-FU. Int J Radiat Oncol Biol Phys 1997; 37:601–14.
- Wallace HJ, Willett CG, Shellito PC, Coen JJ, Hoover HC, Jr. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. J Surg Oncol 1995; 60:122–7.
- Abuchaibe O, Calvo FA, Azinovic I, Aristu J, Pardo F, Alvarez-Cienfuegos J. Intraoperative radiotherapy in locally advanced recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 1993;26:859–67.
- Eble MJ, Lehnert T, Treiber M, Latz D, Herfarth C, Wannenmacher M. Moderate dose intraoperative and external beam radiotherapy for locally recurrent rectal carcinoma. Radiother Oncol 1998;49:169–74.
- Mannaerts GH, Rutten HJ, Martijn H, Hanssens PE, Wiggers T. Comparison of intraoperative radiation therapy-containing multimodality treatment with historical treatment modalities for locally recurrent rectal cancer. Dis Colon Rectum 2001;44:1749–58.
- Pacelli F, Di GA, Papa V, Tortorelli AP, Covino M, Ratto C, et al. Preoperative radiotherapy combined with intraoperative radiotherapy improve results of total mesorectal excision in patients with T3 rectal cancer. Dis Colon Rectum 2004;47:170–9.
- Wiig JN, Tveit KM, Poulsen JP, Olsen DR, Giercksky K-E. Preoperative irradiation and surgery for recurrent rectal cancer. Will intraoperative irradiation (IORT) be of additional benefit? A prospective study. Radiother Oncol 2002; 62:207–13.
- Shoup M, Guillem JG, Alektiar KM, Liau K, Paty PB, Cohen AM, et al. Predictors of survival in recurrent rectal cancer after resection and intraoperative radiotherapy. Dis Colon Rectum 2002;45:585–92.
- Ferenschild FT, Vermaas M, Nuyttens JJ, Graveland WJ, Marinelli AW, van der Sijp JR, et al. Value of intraoperative radiotherapy in locally advanced rectal cancer. Dis Colon Rectum 2006;49:1257–65.
- Dresen RC, Gosens MJ, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, et al. Radical resection after IORT-containing multimodality treatment is the most important determinant for outcome in patients treated for locally recurrent rectal cancer. Ann Surg Oncol 2008;15: 1937–47.
- Rich TA. Intraoperative radiotherapy. Radiother Oncol 1986;6:207–21.
- Dubois JB, Bussieres E, Richaud P, Rouanet P, Becouarn Y, Mathoulin-Pélissier S, et al. Intra-operative radiotherapy of rectal cancer: Results of the French multi-institutional randomized study. Radiother Oncol 2011;98:298–303.
- Masaki T, Takayama M, Matsuoka H, Abe N, Ueki H, Sugiyama M, et al. Intraoperative radiotherapy for oncological and function-preserving surgery in patients with advanced lower rectal cancer. Langenbecks Arch Surg 2008;393:173–80.
- Gunderson LL, Willett CG, Harrison LB, Petersen IA, Haddock MG. Intraoperative irradiation: Current and future status. Semin Oncol 1997;24:715–31.
- Calvo FA, Gomez-Espi M, az-Gonzalez JA, Alvarado A, Cantalapiedra R, Marcos P, et al. Intraoperative presacral electron boost following preoperative chemoradiation in T3-4Nx rectal cancer: Initial local effects and clinical outcome analysis. Radiother Oncol 2002;62:201–6.
- Rahbari NN, Ulrich AB, Bruckner T, Münter M, Nickles A, Contin P, et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: Is there still a chance for cure?Ann Surg 2011;253:522–33.
- Haddock MG, Miller RC, Nelson H, Pemberton JH, Dozois EJ, Alberts SR, et al. Combined modality therapy including intraoperative electron irradiation for locally recurrent colorectal cancer. Int J Radiat Oncol Biol Phys 2011; 79:143–50.
- Skandarajah AR, Lynch AC, Mackay JR, Ngan S, Heriot AG. The role of intraoperative radiotherapy in solid tumors. Ann Surg Oncol 2009;16:735–44.
- Cantero-Munoz P, Urien MA, Ruano-Ravina A. Efficacy and safety of intraoperative radiotherapy in colorectal cancer: A systematic review. Cancer Lett 2011;306:121–33.
- Mirnezami R, Chang GJ, Das P, Chandrakumaran K, Tekkis P, Darzi A, et al. Intraoperative radiotherapy in colorectal cancer: Systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg Oncol 2013;22:22–35.
- Valentini V, Balducci M, Tortoreto F, Morganti AG, De GU, Fiorentini G. Intraoperative radiotherapy: Current thinking. Eur J Surg Oncol 2002;28:180–5.
- Mathis KL, Larson DW, Dozois EJ, Cima RR, Huebner M, Haddock MG, et al. Outcomes following surgery without radiotherapy for rectal cancer. Br J Surg 2012;99:137–43.
- Palmer G, Martling A, Cedermark B, Holm T. A population-based study on the management and outcome in patients with locally recurrent rectal cancer. Ann Surg Oncol 2007; 14:447–54.
- Lim SB, Yu CS, Hong YS, Kim TW, Kim JH, Kim JC. Long-term outcomes in patients with locally advanced rectal cancer treated with preoperative chemoradiation followed by curative surgical resection. J Surg Oncol 2012;106:659–66.
- Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized phase 3 trial comparing preoperative and postoperative chemoradiotherapy with capecitabine for locally advanced rectal cancer. Cancer 2011;117:3703–12.
- Roeder F, Treiber M, Oertel S, Dinkel J, Timke C, Funk A, et al. Patterns of failure and local control after intraoperative electron boost radiotherapy to the presacral space in combination with total mesorectal excision in patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2007; 67:1381–8.
- Sanfilippo NJ, Crane CH, Skibber J, Feig B, Abbruzzese JL, Curley S, et al. T4 rectal cancer treated with preoperative chemoradiation to the posterior pelvis followed by multivisceral resection: Patterns of failure and limitations of treatment. Int J Radiat Oncol Biol Phys 2001;51:176–83.
- Sadahiro S, Suzuki T, Ishikawa K, Fukasawa M, Saguchi T, Yasuda S, et al. Preoperative radio/chemo-radiotherapy in combination with intraoperative radiotherapy for T3-4Nx rectal cancer. Eur J Surg Oncol 2004;30:750–8.
- Kusters M, Valentini V, Calvo FA, Krempien R, Nieuwenhuijzen GA, Martijn H, et al. Results of European pooled analysis of IORT-containing multimodality treatment for locally advanced rectal cancer: Adjuvant chemotherapy prevents local recurrence rather than distant metastases. Ann Oncol 2010;21:1279–84.
- Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355: 1114–23.
- Gerard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol 2006; 24:4620–5.
- Ratto C, Valentini V, Morganti AG, Barbaro B, Coco C, Sofo L, et al. Combined-modality therapy in locally advanced primary rectal cancer. Dis Colon Rectum 2003;46:59–67.
- Diaz-Gonzalez JA, Calvo FA, Cortes J, García-Sabrido JL, Gómez-Espí M, Del Valle E, et al. Prognostic factors for disease-free survival in patients with T3-4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int J Radiat Oncol Biol Phys 2006;64:1122–8.
- Huber FT, Stepan R, Zimmermann F, Fink U, Molls M, Siewert JR. Locally advanced rectal cancer: Resection and intraoperative radiotherapy using the flab method combined with preoperative or postoperative radiochemotherapy. Dis Colon Rectum 1996;39:774–9.
- Willett CG, Shellito PC, Tepper JE, Eliseo R, Convery K, Wood WC. Intraoperative electron beam radiation therapy for primary locally advanced rectal and rectosigmoid carcinoma. J Clin Oncol 1991;9:843–9.
- Nakfoor BM, Willett CG, Shellito PC, Kaufman DS, Daly WJ. The impact of 5-fluorouracil and intraoperative electron beam radiation therapy on the outcome of patients with locally advanced primary rectal and rectosigmoid cancer. Ann Surg 1998;228:194–200.
- Hashiguchi Y, Sekine T, Sakamoto H, Tanaka Y, Kazumoto T, Kato S, et al. Intraoperative irradiation after surgery for locally recurrent rectal cancer. Dis Colon Rectum 1999; 42:886–93.
- Larsen SG, Wiig JN, Dueland S, Giercksky KE. Prognostic factors after preoperative irradiation and surgery for locally advanced rectal cancer. Eur J Surg Oncol 2008;34:410–7.
- Kusters M, Dresen RC, Martijn H, Nieuwenhuijzen GA, van de Velde CJ, van den Berg HA, et al. Radicality of resection and survival after multimodality treatment is influenced by subsite of locally recurrent rectal cancer. Int J Radiat Oncol Biol Phys 2009;75:1444–9.
- Kusters M, Marijnen CA, van der Velde, Rutten HJ, Lahaye MJ, Kim JH, et al. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol 2010;36:470–6.
- Valentini V, Coco C, Rizzo G, Manno A, Crucitti A, Mattana C, et al. Outcomes of clinical T4M0 extra- peritoneal rectal cancer treated with preoperative radiochemotherapy and surgery: A prospective evaluation of a single institutional experience. Surgery 2009;145:486–94.
- Suzuki K, Gunderson LL, Devine RM, Weaver AL, Dozois RR, Ilstrup DM, et al. Intraoperative irradiation after palliative surgery for locally recurrent rectal cancer. Cancer 1995;75:939–52.
- Martinez-Monge R, Nag S, Martin EW. Three different intraoperative radiation modalities (electron beam, high-dose-rate brachytherapy, and iodine-125 brachytherapy) in the adjuvant treatment of patients with recurrent colorectal adenocarcinoma. Cancer 1999;86:236–47.
- Wells BJ, Stotland P, Ko MA, Al-Sukhni W, Wunder J, Ferguson P, et al. Results of an aggressive approach to resection of locally recurrent rectal cancer. Ann Surg Oncol 2007;14:390–5.
- Kanemitsu Y, Hirai T, Komori K, Kato T. Prediction of residual disease or distant metastasis after resection of locally recurrent rectal cancer. Dis Colon Rectum 2010; 53:779–89.
- Salo JC, Paty PB, Guillem J, Minsky BD, Harrison LB, Cohen AM. Surgical salvage of recurrent rectal carcinoma after curative resection: A 10-year experience. Ann Surg Oncol 1999;6:171–7.
- Wiig JN, Larsen SG, Dueland S, Giercksky KE. Preoperative irradiation and surgery for local recurrence of rectal and rectosigmoid cancer. Prognostic factors with regard to survival and further local recurrence. Colorectal Dis 2008; 10:48–57.
- Lindel K, Willett CG, Shellito PC, Ott MJ, Clark J, Grossbard M, et al. Intraoperative radiation therapy for locally advanced recurrent rectal or rectosigmoid cancer. Radiother Oncol 2001;58:83–7.
- Roeder F, Goetz JM, Habl G, Bischof M, Krempien R, Buechler MW, et al. Intraoperative electron radiation therapy (IOERT) in the management of locally recurrent rectal cancer. BMC Cancer 2012;12:592.
- Mohiuddin M, Lingareddy V, Rakinic J, Marks G. Reirradiation for rectal cancer and surgical resection after ultra high doses. Int J Radiat Oncol Biol Phys 1993;27: 1159–63.
- Hashiguchi Y, Sekine T, Kato S, Sakamoto H, Nishimura Y, Kazumoto T, et al. Indicators for surgical resection and intraoperative radiation therapy for pelvic recurrence of colorectal cancer. Dis Colon Rectum 2003;46:31–9.
- Hansen MH, Balteskard L, Dorum LM, Eriksen MT, Vonen B. Locally recurrent rectal cancer in Norway. Br J Surg 2009;96:1176–82.
- Park JK, Kim YW, Hur H, Kim NK, Min BS, Sohn SK, et al. Prognostic factors affecting oncologic outcomes in patients with locally recurrent rectal cancer: Impact of patterns of pelvic recurrence on curative resection. Langenbecks Arch Surg 2009;394:71–7.
- Bedrosian I, Giacco G, Pederson L, Rodriguez-Bigas MA, Feig B, Hunt KK, et al. Outcome after curative resection for locally recurrent rectal cancer. Dis Colon Rectum 2006; 49:175–82.
- Harris GJ, Senagore AJ, Lavery IC, Church JM, Fazio VW. Factors affecting survival after palliative resection of colorectal carcinoma. Colorectal Dis 2002;4:31–5.
- Valentini V, Balducci M, Tortoreto F, Morganti AG, De Giorgi U, Fiorentini G. Intraoperative radiotherapy: Current thinking. Eur J Surg Oncol 2002;28:180–5.
- Lee JH, Kim DY, Kim SY, Park JW, Choi HS, Oh JH, et al. Clinical outcomes of chemoradiotherapy for locally recurrent rectal cancer. Radiat Oncol 2011;6:51.
- Larsen SG, Wiig JN, Emblemsvaag HL, Grøholt KK, Hole KH, Bentsen A, et al. Extended total mesorectal excision in locally advanced rectal cancer (T4a) and the clinical role of MRI-evaluated neo-adjuvant downstaging. Colorectal Dis 2009;11:759–67.
- Kusters M, Holman FA, Martijn H, Nieuwenhuijzen GA, Creemers GJ, Daniels-Gooszen AW, et al. Patterns of local recurrence in locally advanced rectal cancer after intra- operative radiotherapy containing multimodality treatment. Radiother Oncol 2009;92:221–5.
- Valentini V, Aristei C, Glimelius B, Minsky BD, Beets-Tan R, Borras JM, et al. Multidisciplinary rectal cancer management: 2nd European Rectal Cancer Consensus Conference (EURECA-CC2). Radiother Oncol 2009;92:148–63.
