Abstract
Background. Breast cancer is characterized by great molecular heterogeneity demonstrated, e.g. by the intrinsic subtypes. Administration of post-mastectomy radiotherapy (PMRT) does, however, not reflect this heterogeneity. A gene profile (DBCG-RT profile) has recently been developed and validated, and has shown prognostic impact in terms of loco-regional failure and predictive impact for PMRT. Reports have also shown predictive value in terms of benefit of PMRT from intrinsic subtypes and derived approximations. The aim of this study was to examine: 1) the agreement between various methods for determining the intrinsic subtypes; and 2) the relationship between the prognostic and predictive impact of the DBCG-RT profile and the intrinsic subtypes.
Material and methods. Intrinsic subtypes and the DBCG-RT profile was determined from microarray analysis based on fresh frozen tissue from 191 patients included in the Danish Breast Cancer Cooperative Group (DBCG) 82bc trial. Corresponding formalin-fixed, paraffin-embedded tissue was available from 146 of these patients and from another 890 DBCG82bc patients. Estrogen receptor, progesterone receptor, HER2, CK5/6, Ki-67 and EGFR were combined into immunohistochemical approximations of the intrinsic subtypes. Endpoint considered was loco-regional recurrence (LRR).
Results. The DBCG-RT profile identified a group of patients with low risk of LRR and no additional benefit from PMRT among all subtypes. Combining six immunohistochemical markers identified a subgroup of triple negative patients with high risk of LRR and significant benefit from PMRT. Agreement in the different assignments of tumors to the subtypes was suboptimal, and the clinical outcome and predicted benefit from PMRT varied according to the method used for assignment.
Conclusion. The prognostic and predictive information obtained from the DBCG-RT profile cannot be substituted by any approximation of the tumors intrinsic subtype. The predictive value of the intrinsic subtypes in terms of PMRT was influenced by the method used for assignment to the intrinsic subtypes.
Post-mastectomy radiotherapy (PMRT) is currently recommended to breast cancer patients estimated to have a high risk of loco-regional recurrence (LRR) based on a clinico-pathological risk-estimation [Citation1]. The risk-estimation does, however, not sufficiently reflect the heterogeneity in breast cancer, and some patients may not benefit from PMRT but are only risking side effects [Citation2,Citation3]. The extensive examination of the Danish Breast Cancer Cooperative Group (DBCG) 82bc trial, exploring the indication for PMRT in combination with adjuvant systemic treatment in post- and pre-menopausal women with high-risk breast cancer [Citation4,Citation5], has largely contributed to the current knowledge on PMRT, but until recently no subgroup has been revealed with no benefit from PMRT.
In an attempt to individualize the treatment to breast cancer patients, several gene expression profiles have been published, mostly showing prognostic impact in terms of distant metastasis (DM) [Citation6,Citation7], or predictive impact for response to different systemic treatment strategies [Citation6,Citation8]. With the aim to individualize adjuvant radiation therapy, the risk of LRR after mastectomy has also been explored, and recently, the first gene profile (DBCG-RT profile), prognostic in terms of loco-regional control and capable of predicting benefit from PMRT, was derived from the DBCG82bc cohort and independently validated [Citation9]. The DBCG-RT profile separated patients into two risk groups (“Low LRR risk” and “High LRR risk”) and hereby allowed the identification of a subgroup of patients (“Low LRR risk”) with no additional benefit from radiotherapy in terms of LRR. The DBCG-RT profile was found to be independent of clinico-pathological variables.
Over the last decade, it has become increasingly clear that breast cancer is characterized by a heterogeneity that conventional histopathology is not capable of describing. The gene expression profiling study by Perou et al. [Citation10] demonstrated that breast cancer can be classified into intrinsic subtypes (Luminal A, Luminal B, HER2-like, Basal-like, Normal-like) based on 496 genes, and the subtypes were later shown to correlate with clinical outcome [Citation11]. The intrinsic subtypes have been adapted into the terminology of breast cancer and have proven stable across platforms and patient cohorts [Citation12]. A published prediction analysis of microarray (PAM50) [Citation13] uses a minimized set of 50 genes to determine the intrinsic subtypes. The PAM50 method is, however, not standardized and several ways of determining the PAM50 exists [Citation13,Citation14]. Attempts have also been made to approximate the subtypes by combining immunohistochemical markers, primarily estrogen receptor (ER), progesterone receptor (PR) and HER2 [Citation15,Citation16], but also including proliferation marker Ki-67, basal cell markers CK5/6 and EGFR [Citation17–19]. PAM50 and the immunohistochemical approximations use the same terminology as the original centroid correlation-based intrinsic subtypes [Citation11], though it has been questioned if the two gene expression-based methods reliably assign samples into the same subtype [Citation20].
The prognostic and predictive impact of approximations of the intrinsic subtypes has also been studied [Citation15–17], and in general, the “Basal-like” (ER-, PR-, HER2-) and “HER2-like” (ER-, PR-, HER2+) subtypes have been associated with highest LRR rates. The predictive impact of the immunohistochemical subtypes in terms of PMRT was explored in the DBCG82bc cohort [Citation16], and results showed PMRT to significantly reduce LRR probabilities for the “Basal-like” subgroup and for subgroups with luminal features (ER+ and/or PR+). The most striking result was that the largest absolute reduction in LRR rate and largest translation of LRR rate reduction into survival benefit were observed among patients with the most advantageous prognostic features (ER/PR+ and HER2−). The study by Wu et al. [Citation15] also showed significantly reduced LRR rates for patients with Luminal subtypes, when PMRT was given. Similarly, in a study of 128 premenopausal patients from the British Columbia Trial and 87 premenopausal DBCG82b patients an improved LRR- free survival was observed for women with Luminal A tumors, when PMRT was given [The British Columbia trial: HR = 0.12, 95% confidence intervals (CI), 0.01 −0.91; DBCG82b cohort: HR = 0.12(0.14–1.02)] [Citation21].
The aims of this study were to examine: 1) if there is agreement between assignment to the intrinsic subtypes by different gene expression based and two different immunohistochemical combinations (IHC3 and IHC6); and 2) if a gene expression based, or an extended immunohistochemically based approximation of the intrinsic subtypes is superior in predicting the benefit from PMRT than previously described from combination of ER, PR and HER2. Finally, we wanted to examine: 3) if a relationship exists between the predictive impact of the DBCG-RT profile and the intrinsic subtypes with emphasis on the luminal subtypes.
Material and methods
Patient cohort
The DBCG82bc cohort has been described in detail elsewhere [Citation4,Citation5]. In brief, 3083 high-risk breast cancer patients (< 70 years of age) treated with mastectomy and partial axillary dissection were included in the period 1982–1990. The pre-menopausal women (DBCG82b) were randomized to radiotherapy and cyclophosphamide, methotrexate, and fluorouracil (CMF), or CMF alone. The post-menopausal women (DBCG82c) were randomized to radiotherapy and tamoxifen, or tamoxifen alone. Radiotherapy was delivered as an anterior photon field against the supraclavicular, infraclavicular and axillary lymph nodes, and an anterior electron field against the chest wall and intramammary lymph nodes. The intended dose was 50 Gy/25 fractions/5 weeks, or 48 Gy/22 fractions/51/2 weeks [Citation4,Citation5]. A median of seven axillary lymph nodes were removed.
Tissue samples
Fresh frozen tumor (FFT) samples with subsequent successful microarray analysis were available from 191 DBCG82bc patients with a median of seven axillary lymph nodes removed [Citation9]. Corresponding formalin-fixed paraffin embedded (FFPE) samples were available for 146/191 patients. Furthermore, FFPE was available for another 890 DBCG82bc patients with ≥ 7 lymph nodes removed. The 890 patients were part of the study by Kyndi et al. [Citation16]. Fraction of tumor content in the samples was estimated by visual inspection of an HE-section and validated by stereological point counting in FFT samples.
Whole genome array analysis
Extraction of mRNA from FFT and microarray analysis was performed as described in detail by Myhre et al. [Citation22]. The microarray platform used was “Applied Biosystem Human Genome Survey Microarray v2.0” (Applied Biosystem, Foster City, CA, US). Microarray data was log2-transformed, quantile normalized, and filtered using a signal to noise ratio of 3. The data has previously been published (GEO: GSE24117) [Citation22]. The DBCG-RT profile had previously been determined, and the 191 patients separated into 48 “Low LRR risk” patients and 143 “High LRR risk” patients [Citation9]. The intrinsic subtypes (CC) were determined from microarray data from the 191 FFT samples, using the centroid correlation method as described by Sørlie et al. [Citation12]. PAM50 were also determined from microarray based gene expression data following the method as described by Parker et al. [Citation13].
Immunohistochemistry
Tissue microarrays (TMA) had previously been constructed from all FFPE samples using one centrally located core (1 mm in diameter) [Citation23]. Antibody information and FISH probe are stated in Supplementary Table I (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). Immunohistochemical determination of ER and PR as well as HER2 receptor had been performed previously and staining procedures described in detail [Citation23]. Hormone receptors were originally scored according to a 10% threshold, but negative samples were re-scored according to the current 1% threshold. HER2 was scored according to the HercepTest, and equivocal samples (2+) were supplemented with FISH analysis for gene amplification. Tumors were considered as amplified for the ERBB2 gene, if ERBB2/CEN17 ratio ≥ 2.
CK5/6 was considered positive, if any cytoplasmic staining was observed regardless of staining intensity. EGFR positivity was recorded, if any membranous staining of any intensity was observed, and Ki67 if definite nuclear staining of any intensity was observed. For analysis, a 14% cut-off was chosen for Ki-67 [Citation19]. IHC3 were similar to the subgroups by Kyndi et al. [Citation16] except that the 1% cut-off for ER and PR was used. Approximations based on all six immunohistochemical markers (IHC6) were categorized as previously published [Citation17] ().
Table I. Combination of immunohistochemical markers into intrinsic subtypes.
For IHC3 and IHC6, the “HER2” groups are identical. The “Luminal B” group as defined by IHC3 is identical to the “Luminal HER2” group defined by IHC6, whereas the “Luminal A” group as defined by IHC3 is split into a low proliferation group “Luminal A” and a high proliferation group nominated “Luminal B” in the IHC6 terminology. The “basal” group as defined by IHC3 is split into two triple negative groups in IHC6; one expressing basal markers (CK5/6) or EGFR (“Core basal”) and one without basal-like features (“TNP-non-basal”).
Statistical analysis
The endpoint considered was LRR defined as the appearance of local or regional disease (chestwall, axilla, supra/infraclavicular) occurring as an isolated event, or at least one month before DM, or simultaneously with DM within ± 1 month. LRR occurring more than one month after DM was censored at time to DM, and did not count as a LRR. Patients with DM and no LRR were censored at DM-time, and patients with neither DM nor LRR were censored at last date of vital-status/follow-up [Citation4,Citation5]. The closing date for assessment of recurrence and vital status was 1 January, 2012. The potential median observation time was 25.1 years.
A competing risk model was used for calculating cumulative incidence with inclusion of death before LRR or development of DM as competing events. Cumulative incidence probability curves were plotted and tested for differences (Wald test). Cox univariate regression analyses were performed, and assumptions of proportional hazards were tested graphically using log-minus-log plots. Positive agreements were used for evaluating relationship between the different approaches of assigning the patients into subtypes, and were calculated as number of positive samples that agree by two methods divided by the number of positive samples by the chosen reference standard. Level of significance was 5%, and all estimated p-values were two-sided. Statistical calculations were performed using STATA version 11.2 (StataCorp, College Station, TX, USA) and R (Development Core Team, 2011).
Results
Intrinsic subtypes
Intrinsic subtypes was determined by the original CC method in 190/191 FFT samples (one missing value), and by PAM50 in all 191 FFT samples. IHC3 was determined in all 1036 (146 + 890) FFPE samples. IHC6 was determined in 980/1036 (137/146 + 843/890) FFPE samples, but assignment to Luminal A or Luminal B subtype failed for 9/146 and 44/890 ER positive/HER2 negative samples due to missing Ki-67 values, and 3/890 samples failed to be classified due to missing EGFR and/or CK5/6 status in combination with triple negativity. The median tumor area fraction was 50% (range: 5–85%) in the 191 FFT samples, 60% in the corresponding 146 FFPE samples (range: 5–100%) and 70% (range: 5–100%) in the 890 FFPE samples. Samples assigned to the Normal-like subtype by either CC or by PAM50 had a significantly lower content of median tumor epithelial cells judged from the frozen HE-sections (35% and 30%, respectively; range: 5–70%) as compared to the content in the FFT samples (p < 0.0001). Luminal A was the most frequent subtype, constituting 38%, 27%, 59% and 34% of the 191 examined samples for CC, PAM50, IHC3 and IHC6, respectively.
In the group of 890 patients, 64% (569/890), 9% (83/890), 12% (103/890) and 15% (135/890) was determined by IHC3 as “Luminal A”, “Luminal B”, “HER2-like” and “Basal-like”, respectively. Based on IHC6, 49% (417/843), 11% (96/843), 16% (131/843), 11% (90/843), 10% (86/843) and 3% (23/843) of the 843/890 patients was determined as “Luminal A”, “Luminal HER2”, “Luminal B”, “HER2-like”, “Core basal” and “TNP-non-basal”, respectively.
Agreement between molecular- and immunohistochemical assignment to the intrinsic subtypes
Agreements in assignment to the intrinsic subtypes as defined by CC, PAM50, IHC3 and IHC6 were determined and are stated as absolute numbers in , and in percentages for subtypes with identical terminology in Supplementary Table II (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). The overall positive agreement was 63% (95% CI 55–70%) between CC and PAM50 (119/190 samples). After excluding the Normal-like subtype, which cannot unequivocally be defined by IHC, there was 69% (59–76%) agreement between CC and IHC3 (83/145 samples) and 47% (38–56%) between PAM50 and IHC3 (61/130 samples). Overall positive agreements including IHC6 was not calculated, since IHC6 introduces new categories for the intrinsic subtypes overlapping the original categorization. The agreement in assignment to the various subtypes differed substantially, with most consistent allocation to the Luminal A and/or Basal-like subtypes (Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580).
Table II. Distribution of patients according to DBCG-RT profile and intrinsic subtypes.
Using CC as the reference standard, Basal-like was the subtype most reliably reproduced by PAM50 ( and Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). The majority of the 71 samples that showed discrepancy between CC and PAM50 was found to comprise 26/71 (37%) samples determined as Luminal A by CC, but as Luminal B according to PAM50. None of the samples originally determined as Basal-like according to CC was classified as being luminal according to PAM50.
Luminal A and Basal-like subtypes were the subtypes most reliably approximated by IHC3, when CC was considered the reference standard (Supplementary Table II available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). Adding Ki-67, EGFR and CK5/6 (IHC6) led to a poorer determination of the Luminal A tumors, and the overall positive agreements did not improve significantly when using IHC6 instead of IHC3 in comparison to CC.
In general, the agreements between PAM50 and IHC3 were poorer than as between CC and IHC3, when PAM50 was chosen as a non-reference standard. Especially the positive agreement for the Luminal B subtype was found to be very poor. This agreement was improved when comparing PAM50 and IHC6, but the agreement for Luminal A declined simultaneously. For IHC6, only positive agreements for Luminal A, Luminal B and HER2 were calculated, since these are the only subtypes that fitted a category within the IHC6.
Prognostic value of intrinsic subtypes
In Supplementary Figure 1A–D (available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580), the clinical outcome in terms of local control can be seen for the subgroup of 94/191 patients treated with systemic treatment only (No PMRT). When using Luminal A as a reference in univariate Cox analyses, none of the subtypes as determined by CC or PAM50 was found to be significantly associated with risk of LRR, and IHC6 did not add prognostic information in comparison to IHC3. However, PAM50 showed a diverging outcome between the Luminal subtypes than the other approaches with a surprisingly better local control for Luminal B than Luminal A (Supplementary Figure 1D available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580).
The prognostic value of IHC3 and IHC6 was further tested in the larger set of 453/890 DBCG82bc non-irradiated patients with only FFPE available (). Additional prognostic information was achieved by IHC6 in the group of triple negative tumors. The “Core basal” group expressing either EGFR or CK5/6 showed a lower risk of LRR than the “TNP-non-basal” group, and in a univariate analysis the “TNP-non-basal” group was found to have a significantly higher risk of LRR [Unadjusted HR: 3.11(1.55–6.22)] compared to Luminal A, whereas the “Core basal” group did not [Unadjusted HR: 0.82(0.41–1.64)].
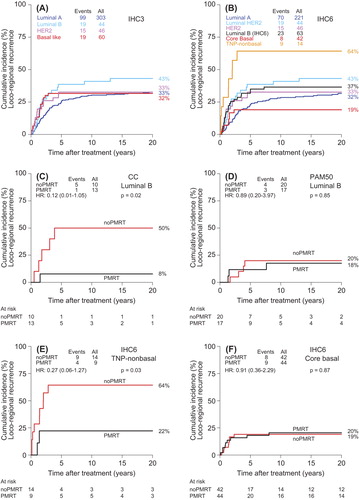
Figure 1. Plots of cumulative incidence proportion of loco-regional recurrence (LRR). (A) IHC3 determined from combination of the expression of estrogen receptor (ER), progesterone receptor (PR) and HER2 in the subgroup of 453/890 patients treated with systemic treatment only and no post-mastectomy radiotherapy. (B) IHC6 determined from combination of ER, PR, HER2, Ki-67, CK5/6 and EGFR in the same group of 453 non-irradiated patients. IHC6 shows separation of the triple negative group into a group with no basal-like features (“TNP-non-basal”) and high risk of LRR, and a group with expression of CK5/6 and/or EGFR (“Core Basal”) and low risk of LRR. (C,D) Luminal B subtypes determined by centroid correlation method (CC) (C) and PAM50 (D). The group of patients designated as Luminal B by PAM50 shows no benefit from PMRT in contrast to the Luminal B tumors as determined by CC. (E,F) The two groups of triple negative tumors determined by IHC6 in the 854 DBCG82bc patients (E) “TNP-non-basal” and (F) “Core basal”, showing significantly different response to PMRT.
Predictive value regarding PMRT of the intrinsic subtypes
The predictive value in terms of benefit from PMRT was influenced by the approach used for determining the intrinsic subtypes. For Luminal A tumors as determined by the various approaches a significant benefit from PMRT in terms of LRR rate reduction could be seen, as previously described [Citation16,Citation21] (Supplementary Figure 2A–D, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). The results, however, showed a benefit from PMRT in the Luminal B tumors defined by CC (), but not by PAM50 ().
Additional predictive information was also achieved by IHC6 in the triple negative group of patients. The 25/890 patients determined by IHC6 to have “TNP-non-basal” tumors showed benefit from PMRT in terms of LRR (), whereas the 86/890 patients with “Core basal” tumors showed no additional LRR reduction when PMRT was given (). This indicated a possible radioresistancy in the presence of EGFR or CK5/6 expression. The number of patients and events was, however, limited and conclusions must be taken with caution.
The predictive value in regard to PMRT of the DBCG-RT profile in relation to the intrinsic subtypes
The DBCG-RT profile has previously, in the same series of patients, been described and validated as having prognostic value in terms of local control and predictive impact in regard to benefit from PMRT [Citation9]. Among the 94/191 non-irradiated patients treated with systemic treatment alone, the DBCG-RT profile identified two groups (“Low LRR risk” and “High LRR risk”) with significantly different prognosis regarding LRR risk [57% vs. 8% at 20 years; p < 0.0001; adjusted HR = 0.07 (0.02 to 0.30)]. The DBCG-RT profile was further predictive of PMRT that could be seen to reduce LRR risk in the “High LRR risk” patients [57% vs. 12% at 20 years; p < 0.0001; adjusted HR = 0.17 (0.08 to 0.34)] whereas the “Low LRR risk” patients experienced no additional benefit from radiotherapy [8% vs. 9% at 20 years; p = 0.93; adjusted HR = 1.13 (0.14–9.15)]. The DBCG-RT profile was modified to RT-qPCR and FFPE from 146/191 patients, and subsequently, the FFPE/RT-qPCR modified DBCG-RT profile independently validated in 112 additional DBCG82bc patients.
A significant difference in distribution of the intrinsic subtypes between the “Low LRR risk” and “High LRR risk” groups could be seen for CC, PAM50 and IHC3 in the 191 patients (Supplementary Table III, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). In general, there was more Luminal A tumors in the “Low LRR risk” group, and more Basal and HER2 subtypes in the “High LRR risk” group. A large fraction of Luminal A tumors was, however, also found to have a “High LRR risk” profile ( and Supplementary Figure 2E–H available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). The risk groups defined by the DBCG-RT profile were not exclusively confined to specific subtypes, and a subgroup of patients with “Low LRR risk” and no benefit from PMRT could be identified even among the apparently radiosensitive Luminal A subtype as determined by all four methods ( and Supplementary Figure 2I–L available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). Among the Basal subtype in CC and “TNP-non-basal” group in IHC6, all patients were found to have a “High LRR risk” profile ().
In the “Low LRR risk” group, the few LRRs were primarily found among the frequent Luminal A tumors (Supplementary Table III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). In the “High LRR risk” group, LRR could be found among all subtypes, except among the “TNP-non-basal” group.
In the PAM50 determined Luminal B subtype, 21 patients had a “High LRR risk” profile but among this limited number of patients there was no significant difference in the LRR rate between patients treated with PMRT or not (Supplementary Figure 3, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580). For all other subtypes regardless of method for assignment, a reduction in the risk of LRR by PMRT could be found in the “High LRR risk” group (Supplementary Table III available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.925580).
These results indicated that the previously described prognostic and predictive effect of the DBCG-RT profile was independent of intrinsic subtype, regardless of which method was used for determining the subtypes.
Discussion
In this study, we found that the described prognostic and predictive information of the DBCG-RT profile could not be substituted by the intrinsic subtypes. The assignment to the intrinsic subtypes was not identical when using various approaches and the subtypes seemed to hold diverse information in terms of benefit from PMRT.
PAM50 as well as a number of immunohistochemical combinations use the same nomenclature as the original intrinsic subtypes as described by Perou et al. [Citation10], even though previous observations [Citation20] have questioned the consistency in the assignment to the intrinsic subtypes. The present study supports this lack of concordance in assignment. The original intrinsic gene list of 496 genes [Citation10] encompassed genes showing a significantly greater variation in expression between different tumor samples than between paired samples. The 50 genes in PAM50 includes relatively more proliferation genes than the original intrinsic gene list, and this is likely contributing to the discordance in the assignment between the two methods, especially for the luminal subgroups considered to differ on the basis of high/low proliferation. In the present study, a fraction of tumors determined as Luminal A with the original CC based method was indeed designated as Luminal B with PAM50 in concordance with the differences in proliferation-based genes.
The immunohistochemical subtypes relates to expression of very few proteins occurring from gene products that may have been post-translationally modified, and they represents very basic approximations of the gene expression-based subtypes. A previous study have shown that immunohistochemistry does not adequately identify PAM50 [Citation24], and only 71% of HER2-like tumors with a high ERBB2 gene expression, defined by PAM50, were found to be HER2 positive by immunohistochemistry and/or in situ hybridization. The study also showed that only 75% of ER negative tumors were classified into non-luminal subtypes (HER2 and Basal-like) [Citation24].
The terms Luminal A, Luminal B, Normal-like, Basal-like and HER2 are, therefore, not mutually exclusive when defined by different methods, and the terminology should be used with caution and by referring to which method they have been defined from.
In this study, the prognostic value in terms of local control and predictive value in regard to benefit from PMRT of the intrinsic subtypes varied with the method used to determine the subtypes. In comparison to IHC3 [Citation16], IHC6 was found to add information by defining two distinct groups of triple negative tumors with significantly different prognostic outcome in terms of LRR and different benefit from PMRT. The “Core basal” group expressing either EGFR or basal cytokeratins CK5/6 was found to have a low risk of LRR and no significant benefit from PMRT, indicating a radioresistancy possibly related to EGFR.
The CC did not add prognostic or predictive information in comparison to IHC3. PAM50 was found to display diverse prognostic information for the luminal subtypes in comparison to the three other methods. Furthermore, no benefit from PMRT could be documented for patients with PAM50 determined Luminal B tumors; an observation that has also been seen in the British Columbia Trial (72% vs. 44%, p = 0.41) [Citation21]. The patients showed a fairly high risk of LRR and the findings could indicate that patients with a “High LRR risk” profile and a PAM50-based Luminal B subtype may benefit from a more extensive surgical procedure in order to avoid LRR. The results from both studies must, however, be taken with caution, since they are based on small numbers of patients. The observed difference in clinical outcome by PAM50 for the luminal subgroups in comparison to the other approaches can perhaps be related to the procedures for normalization of array datasets [Citation25]. In addition, differences in the study population (i.e. proportion of ER-positive tumors) may also affect the distribution of the subtypes [Citation25]. It must also be emphasized that, PAM50 is not standardized and various methods exists for determining the subtypes by PAM50 [Citation13,Citation14].
When examining the relationship between the prognostic value of the DBCG-RT profile and the intrinsic subtypes, the present study showed a correlation between the good prognosis group of the DBCG-RT profile (“Low LRR risk”) and the Luminal A subtypes that, most consistently, was shown to have the best local control. The poor prognosis group of the DBCG-RT profile (“High LRR risk”) was further found to correlate with the non-luminal, ER negative tumors (Basal-like and HER2-like) regardless of how these subtypes were determined. This non-surprising finding indicates that the DBCG-RT profile and the intrinsic subtypes to a large degree identify the same tumors as being locally aggressive or not.
The predictive value of the DBCG-RT profile stratified by subtype seemed, however, to be in contrast with the studies by Kyndi et al. [Citation16], and Laurberg et al. [Citation21]. In the present study, the two risk groups (“Low LRR risk” and “High LRR risk”) could be identified among all the subtypes, and a subgroup of patients with no benefit from PMRT could be identified by the DBCG-RT profile even among the Luminal A subtype as determined by all four approaches. The results from the analysis of the DBCG-RT profile in relation to intrinsic subtypes contribute to the conclusion that the DBCG-RT profile describes features other than ER- and HER2 positivity/negativity, and that the information, especially in terms of prediction of benefit from PMRT, cannot merely be substituted by determination of the tumors intrinsic subtype. It further indicates that the DBCG-RT profile has an even more refined ability of contributing to an individualized loco-regional treatment/radiotherapy than the intrinsic subtypes. The DBCG-RT profile seems to have the advantage in comparison to clinico-pathological parameters and intrinsic subtype determination that it identifies a subgroup of patients in which PMRT can be safely omitted.
In conclusion, the present study emphasizes that the nomenclature of the original intrinsic subtypes is used to describe subgroups of tumors that are not mutually exclusive, when defined by the different approaches, and that these subgroups produces different information on local control and prediction of benefit from PMRT. Furthermore, the prognostic and predictive information of the DBCG-RT profile could not be substituted by determination of the intrinsic subtypes, as defined by either gene expression or immunohistochemistry.
Supplementary Tables I–III and Figures I–III
Download PDF (195.9 KB)Acknowledgments
The study was financially supported by CIRRO – The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research; The Danish Council for Independent Research | Medical Sciences; The Danish Cancer Society; and Aarhus University. The funding sources of the study have played no role in the design of the study design; in collection, analysis or interpretation of the data; in the writing of the manuscript or in the decision to submit the manuscript for publication.
Declaration of interest: Trine Tramm, Simen Myhre, Jan Alsner, Therese S rlie and Jens Overgaard hold a patent on the presented DBCG-RT profile (international patent publication no. WO 2013/132354 A2). The authors report no other conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- EBCTCG Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet Epub 2014 Mar 19.
- Grantzau T, Mellemkjaer L, Overgaard J. Second primary cancers after adjuvant radiotherapy in early breast cancer patients: A national population based study under the Danish Breast Cancer Cooperative Group (DBCG). Radiother Oncol 2013;106:42–9.
- Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98.
- Overgaard M, Jensen MB, Overgaard J, Hansen PS, Rose C, Andersson M, et al. Postoperative radiotherapy in high- risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353:1641–8.
- Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337:949–55.
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen- treated, node-negative breast cancer. N Engl J Med 2004; 351:2817–26.
- van‘t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002;415:530–6.
- Chang JC, Wooten EC, Tsimelzon A, Hilsenbeck SG, Gutierrez MC, Elledge R, et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 2003;362:362–9.
- Tramm T, Mohammed H, Myhre S, Kyndi M, Alsner J, Børresen-Dale A, et al. Development and validation of a gene profile predicting benefit of post-mastectomy radiotherapy in high risk breast cancer patients: A study of gene expression in the DBCG82bc cohort. EJC Supplements 2013;11:Abstract 1856
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52.
- Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869–74.
- Sørlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 2003;100:8418–23.
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
- Haibe-Kains B, Schroeder M, Bontempi G, Sotiriou C, Quackenbush J. genefu: Relevant functions for gene expression analysis, especially in breast cancer. R package, version 1.14.0.
- Wu SG, He ZY, Li Q, Li FY, Lin Q, Lin HX, et al. Predictive value of breast cancer molecular subtypes in Chinese patients with four or more positive nodes after postmastectomy radiotherapy. Breast 2012;21:657–61.
- Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol 2008;26:1419–26.
- Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol 2010;28:1684–91.
- Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 2004;10:5367–74.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009; 101:736–50.
- Weigelt B, Mackay A, A'hern R, Natrajan R, Tan DS, Dowsett M, et al. Breast cancer molecular profiling with single sample predictors: A retrospective analysis. Lancet Oncol 2010;11:339–49.
- Laurberg T, Tramm T, Gelmon K, Alsner J, Leung S, S rlie T, et al. In two independent randomized trials young high risk breast cancer patients with luminal A tumors had benefit from postmastectomy radiation therapy. EJC Supplements 2013;11:Abstract 1850.
- Myhre S, Mohammed H, Tramm T, Alsner J, Finak G, Park M, et al. In silico ascription of gene expression differences to tumor and stromal cells in a model to study impact on breast cancer outcome. PLoS One 2010;5:e14002.
- Kyndi M, Sorensen FB, Knudsen H, Overgaard M, Nielsen HM, Andersen J, et al. Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of DBCG 82 b & c. Acta Oncol 2008;47: 591–9.
- Bastien RR, Rodriguez-Lescure A, Ebbert MT, Prat A, Munarriz B, Rowe L, et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med Genomics 2012;5:44.
- Lusa L, McShane LM, Reid JF, De Cecco L, Ambrogi F, Biganzoli E, et al. Challenges in projecting clustering results across gene expression-profiling datasets. J Natl Cancer Inst 2007;99:1715–23.
