Abstract
Background. To assess the temporal patterns of late gastrointestinal (GI) and genitourinary (GU) radiotherapy toxicity and resolution rates in a randomised controlled trial (All-Ireland Cooperative Oncology Research Group 97-01) assessing duration of neo-adjuvant (NA) hormone therapy for localised prostate cancer.
Material and methods. Node negative patients with > 1 of: PSA > 20 ng/mL, Gleason score ≥ 7, and stage T3 or more, were included. Follow-up, including toxicity assessment, was three-monthly in the early stages and yearly thereafter.
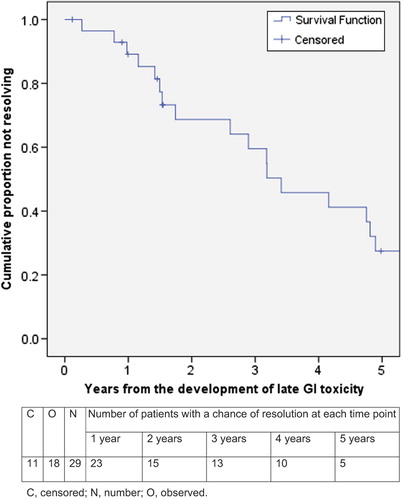
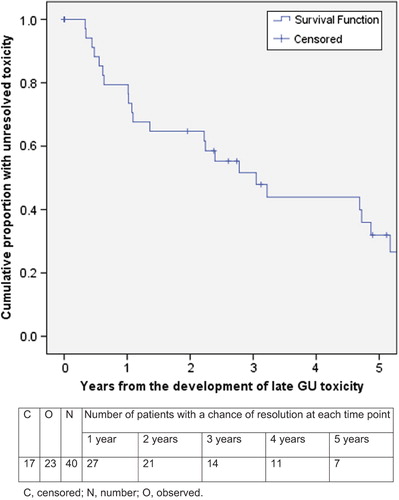
Results. Median follow-up from the end of RT was 6.8 years. In the interval between 90 days following the end of RT and the last toxicity assessment, GI and GU toxicity (any grade) was found in 50% and 51% of 240 and 241 patients, respectively. For those who did develop toxicity, the median time from end of RT until the first development of any grade GI or GU toxicity was 1.2 years and 1.6 years, respectively, whilst median time to final resolution was 1.6 years and 2.2 years, respectively. Grade 2 (G2) or greater GI and GU toxicity occurred in 29 (12.1%) and 40 (16.6%) patients, respectively. The proportion with unresolved G2 + GI and GU toxicity was 89% and 79%, respectively, in year 1, 69% and 65% in year 2, 59% and 52% in year 3 and 27% and 32% in year 5.
Conclusion. Long-term toxicities continue to occur many years after NA hormone therapy and RT. The rate of occurrence does not appear to reduce within the time frame during which our patients were followed. The percentage of patients suffering from G2 + toxicity at any time is however low. Resolution of these toxicities continues for the duration of the follow-up.
Selecting optimal therapy for localised prostate cancer presents a complex choice for physicians and their patients. Efficacy and toxicity are key considerations in making that choice. There is considerable literature describing risk factors for the development of long-term radiotherapy (RT)-induced toxicities and the overall occurrence of these toxicities. The time course of occurrence, evolution and resolution of gastrointestinal (GI) and genitourinary (GU) toxicities are less frequently described [Citation1–5]. These temporal patterns of toxicities may be valuable in helping doctors and patients in selecting the most appropriate intervention and therapy if any is required.
This paper reports the cumulative incidence and temporal evolution of treatment related GI and GU toxicities in prostate cancer patients who were included in a randomised controlled trial (RCT) treated with localised three-dimensional (3D) RT and either four-months (arm 1) or eight-months (arm 2) neo-adjuvant hormonal therapy (NAHT). These data may be used to advise patients about the frequency and projected time course of their expected GI and GU toxicities and may influence decision making regarding intervention versus conservative management.
Material and methods
Patient population
Between 1997 and 2001, 276 patients with intermediate- and high-risk adeno-carcinoma of the prostate were randomised into a phase III RCT. The trial compared biochemical failure after four months and eight months of NAHT followed by RT [Citation6]. The eligibility criteria excluded patients with established nodal disease or metastases. Patients met one or more of the following criteria: PSA > 20 ng/mL, Gleason score ≥ 7, and stage T3 or more. All patients had a Karnofsky performance status (KPS) of ≥ 70. Exclusion criteria included previous treatment for prostate cancer (other than transurethral resection of the prostate), bilateral orchidectomy, prior ADT for prostate cancer, prior malignancies (other than non-melanoma skin cancer), and uncontrolled severe medical illnesses.
The design, objectives, patient eligibility criteria, treatment methods, statistical considerations and main trial outcome have been reported previously [Citation6].
Treatment: Neo-adjuvant hormonal therapy four versus eight months
HT consisted of monthly intramuscular injections of the luteinising hormone-releasing hormone agonist triptorelin (Decapeptyl) 3.75 mg once each month and oral flutamide (Drogenil) anti-androgen tablets (250 mg three times daily).
Radiotherapy modalities
3DCRT was used (70 Gy in 35 fractions by a 3-field technique). The last month of hormonal therapy was used to simulate and plan the radiation. The patients were positioned supine and underwent computed tomography (CT) planning using 0.5-cm slices through the pelvis. The rectum and bladder were delineated with an external contour only. The target volume was drawn by placing a 1-cm margin around the entire prostate and seminal vesicles. This margin was reduced to 0.5 cm in the region of the anterior rectal wall. Treatment was prescribed to an isodose that completely encompassed the target. The maximal permitted hot spot within the target volume was 110%. The dose-volume constraints were that no part of the rectum should receive > 74 Gy; not > 30% of the rectum should receive 100% of the dose; and not > 50% of the bladder should receive 100% of the dose. Multiple fields with customised blocking were used. If necessary, a cone down was performed after 50 Gy (excluding the superior portions of the seminal vesicles from the target volume) to limit the dose to the small bowel to 50 Gy.
Patient follow-up
Follow-up was monthly for the first year, three monthly between years 2 and 3, and yearly thereafter. It included a medical history, rectal examination, PSA blood test and toxicity assessment. GI (non-specific bowel) and GU toxicity was graded using RTOG-EORTC criteria [Citation7]. Morbidity data were recorded at baseline, weekly during RT and at each follow-up visit, by physician-directed questions.
Statistical analyses
The worst severity toxicity documented was considered the final toxicity, even if the complication resolved later on. Final resolution of a G2 morbidity meant symptoms ceased to exist, i.e. were deemed to be G0 and no further toxicity was recorded up to last assessment. The prevalence was defined as the number of cases of graded toxicity at a given time. The date of the toxicity event was taken as the date of the first documentation of the highest grade for each patient. Times to acute toxicity were calculated from the start date of EBRT to the toxicity event or to the date of last assessment up to 90 days following the end of RT for each patient. Times to late toxicity were calculated from 90 days post-EBRT to the toxicity event or date of last assessment. Unresolved acute toxicities were considered to be new late toxicities. Times to final resolution were calculated from the date of the toxicity event to the date of final resolution or date of last assessment.
To assess the time behaviour of symptoms, toxicity grades are given at yearly intervals for seven years after treatment. These interval toxicity grades resulted from the worst symptom observed within each of the periods. Patients who did not attend for follow-up as required by the study or who were not assessed for toxicity because of disease progression were therefore excluded from relevant intervals. Data were not available for detailed toxicities because of the morbidity scale used so, e.g. only GI toxicity is reported here and not rectal bleeding.
The Kaplan-Meier method was used to estimate times to both the toxicity event and to final resolution [Citation8]. The Cox proportional hazards model [Citation9] was used to assess the independent impact of all potentially important explanatory variables (age group, duration of neo-adjuvant hormones, acute toxicity, KPS and T-stage) on late toxicity. The influences of age group, duration of neo-adjuvant hormones, acute toxicity, KPS and T-Stage were assessed. All statistical tests were two-sided and assessed for significance at the 0.05 level. SPSS statistical software version 20.0 (SPSS, Chicago, IL, USA) was used for all analyses.
Results
As previously published [Citation6,Citation10], no significant difference was observed in biochemical failure- free survival, prostate cancer-specific survival, overall survival or toxicity between the four- and eight-month arms.
Toxicity data for 247 patients were analysed for this study (Supplementary Figure 1, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927072). Two hundred and forty-one patients had data for late toxicity. gives the pre-treatment characteristics of these patients. Only three patients had a baseline documented urinary co-morbidity.
Table I. Randomised trial ICORG 97-01: Neo-adjuvant hormone therapy prior to radiation. Pre-treatment characteristics*.
Acute GI and GU toxicity
In the interval between the start of RT and 90 days following the end of RT, GI and GU toxicity (any grade) was found in 127 (52%) and 198 (81%), respectively of 244 patients. G2 + acute GI and GU toxicity was 45 (18%) and 84 (34%), respectively. No patient developed grade 3 or higher GI toxicity. Fourteen (7%) patients developed grade 3 (10 patients in arm 2), and one (0·5%) patient in arm 2 developed grade 4 GU toxicity. For those who developed toxicity, the maximum GI and GU toxicity in this interval developed after the end of RT in 29 (23%) and 48 (24%), respectively of patients.
Late GI toxicity: Rate and temporal trend
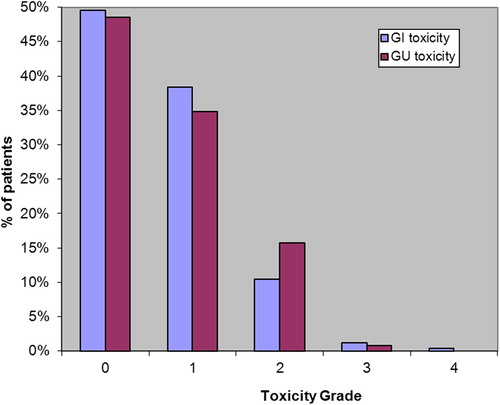
In the interval between 90 days following the end of RT and the latest follow-up GI toxicity (any grade) was found in 121 (50%) patients (). G2 or greater GI toxicity was 29 (12.1%). Four patients (1.7%) had grade 3 or higher GI toxicity (all in the 8-month arm).
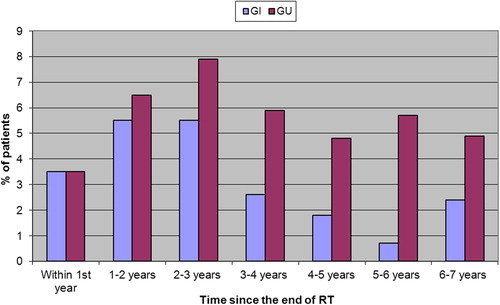
Prevalence of GI symptoms showed fluctuation with 65 (28.5%) patients experiencing maximum GI toxicity within the first year after the end of RT. and 39 (20.6%) patients between three- and four years post-RT. Prevalence of GI symptoms G2 + showed fluctuation with maximum values between one and three years after the end of RT (; Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927072).
At five years the cumulative risk (hazard) of G2 + GI toxicity was 16%. The five-year risk of developing G2 and G3 GI toxicity was 13.3% and 2.4%, respectively. For the 121 who did develop GI toxicity, the median time from end of RT until the first development of any grade GI toxicity was 1.2 years.
Late GU toxicities: Rate and temporal trend
In the interval between 90 days following the end of RT and the latest follow-up GU toxicity (any grade) was found in 124 (51%) patients. G2 + late GU toxicity was 16.6%; higher than that for G2 + late GI toxicity. Two patients (1%) developed Grade 3 GU toxicity.
Prevalence of GU symptoms showed more fluctuation than GI symptoms with 26% (60 patients) experiencing maximum GU toxicity within the first year of finishing RT, then decreasing to 19.9% (43 patients) in year 1–2, but rising again to 24% (34 patients) 5–6 years post-RT. Prevalence of GU symptoms G2 + was lowest within the first year post-RT and the highest 2–3 years post-RT ().
At five years the cumulative risk of G2 + GU toxicity was 20%. For the 124 who did develop GU toxicity, the median time from end of RT until the first development any grade GU toxicity was 1.6 years.
Resolution
Symptoms of late toxicity were not stable over time. Toxicity sometimes resolved for a number of follow-up visits but then appeared again or even worsened at a later visit.
Late GI and GU toxicity (any grade) had final resolution in 94 (78%) and 82 (66%) men, respectively. Median time to final resolution of GI and GU toxicity (any grade) was 1.6 years and 2.2 years, respectively ( and ). A shorter time to final resolution was seen for grade 1 GI and GU toxicities (median 1.1 and 1.6 years, respectively) in 76/92 (83%) and 59/84 (70%) patients, respectively, as opposed to G2 + toxicities with a median time to final resolution of 3.4 and 3.0 years, respectively. The proportion of unresolved G2 GI and GU toxicities, respectively, were 89% and 79% at one year, 69% and 65% at two years, 59% and 52% at three years and 27% and 32% at five years. All three patients with grade 3 GI toxicity (all in arm 2) resolved (at 1.5, 2.9, and 3.2 years). The one instance of grade 4 GI toxicity in arm 2 did not resolve. Of the two patients with grade 3 GU toxicities, there was resolution in only one patient, at 2.8 years.
Twelve patients (48%) with G2 late GI toxicity had had G1 acute GI toxicity (). Acute GI toxicity (p = 0.008) was an independent predictor of late GI toxicity (). The estimated hazard or risk of late GI toxicity increases by 1.8 and 1.7 times, respectively, for those with grades 1 and 2 acute GI toxicity compared to those with no acute GI toxicity.
Table II. Acute by late GI and GU toxicity.
Table III. Cox regression analysis of late bowel toxicity (n = 253).
Sixteen patients (42%) with G2 late GU toxicity had had G1 acute GU toxicity (). Acute GU toxicity (p = 0.015) was an independent predictor of late GU toxicity (). Initial KPS was also predictive of late GU toxicity (but not GI toxicity). The estimated hazard or risk of late GU toxicity increases by 1.9 times for those with a KPS of 70 or 80 compared with those with a KPS of 90–100. On multivariate analysis, KPS remained as significant. The estimated hazard or risk of late GU toxicity increases by 1.34 times for those with a KPS of 70–80 compared to those with a KPS of 90–100 adjusting for acute toxicity. The estimated hazard or risk of late GU toxicity increases by 1.3 times for those with acute GU toxicity compared to those with no acute GU toxicity, adjusting for initial KPS.
Discussion
Late toxicity
The overall incidence of late G2 + GI and GU toxicity was 12.1% and 16.6%, respectively. Our figures are similar to those reported by the MRC dose-escalation trial [Citation11,Citation12] where at two years 15% of patients had G2 + GI toxicity and 17% had G2 + GU toxicity (). We also showed slightly more patients with Grade 1 GI than GU toxicity and slightly more G2 + GU than GI toxicity.
Table IV. Cumulative incidence of late RTOG grade ≥ 2 toxicity in five randomized trials.
Our study supports previous reports that acute GI and GU toxicities were independent predictors of late toxicity. Of note, patients’ KPS was an independent predictor of late GU toxicity. In a study of 973 patients, Jereczek-Fossa [Citation13] et al. reported that any grade late GU toxicity was seen in 36.5% of patients who had experienced acute urinary symptoms, compared with 51% in our study. In addition, a higher grade of acute GU toxicity was predictive for a higher grade of late GU toxicity [Citation13]. In the Dutch Multicentre trial [Citation14–16], the authors observed that late effects were a direct consequence of the initial tissue injury. Late urinary toxicity has also been linked independently to previous history of a TURP and radiation doses higher than 70 Gy [Citation17].
One weakness of our study is the use of the RTOG-EORTC grading criteria. The RTOG-EORTC late radiation morbidity score does “not cover the range of radiation morbidity and uses general definitions on broad categories” [Citation18]. The NCI Common Terminology Criteria for Adverse Events (CTCAE) describes side effects more completely than the RTOG-EORTC scale. Another weakness is the use of physician-directed questions. Several studies have shown that physicians systematically under-report patients’ symptoms [Citation19].
Time course of development
The majority of patients who experienced G1 or higher GI and GU toxicity did so within the first two years post-RT. This is similar to studies by Zelefsky [Citation1], Karlsdottir [Citation4] and Huang [Citation5] where the median time to development of late GI toxicity is 12–30 months. As mentioned above median time to development of toxicity was similar between GI and GU groups in our cohort. Of those that developed toxicity, the median time to development was longer for those who had GU toxicity than GI toxicity (1.6 years vs. 1.2 years). Similar to our study, Zelefsky et al. noted that GU toxicities tended to occur at a later stage compared with GI toxicity (30 months vs. 17 months) [Citation1].
Zietman [Citation20] et al. found that most late G2 + GI morbidity was seen by three years, however, GU morbidity continued to accumulate. At three years, the actuarial risk of a GU event of G2 or higher was 15% (conventional RT) and 13% (high-dose), increasing to 19% and 18% by five years. Capp et al. [Citation21] analysed data from self-assessment questionnaires from patients who were treated within the randomised trial of NAHT and radiation therapy and reported a gradual increase of GI urgency over time with almost 15% of patients affected at 4–5 years.
Our patients were however treated 14–17 years ago. New techniques such as intensity-modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT) spare surrounding organs and tissue and may give less or different side effects. Thus, the results from this study may only be valid for CRT.
Prevalence
As previously stated, the prevalence of GI and GU symptoms showed fluctuation with maximum values within the first year post-RT. The prevalence of grades 2 + GI and GU symptoms, based on smaller numbers and so possibly the result of random variation in the reporting of side effects, also showed fluctuation, with maximum values between 1 and 3 and 2–3 years, respectively, post-RT. This fluctuation seen in our group of patients does not appear to be commonly cited in the literature. Prevalence rates of GI toxicity have been reported with maximum values ranging from 1 to 4 years post-RT [Citation2,Citation3,Citation5]. Researchers from the University of Chicago [Citation3] analysed a group of patients who received a dose of 60–74 Gy for prostate cancer. The numbers of patients with grade 2 and 3 GI toxicity were highest at 3–4 years from the end of RT (2.9%) and dropped to 1.5% at five years after treatment. Karlsdottir et al. [Citation4] reported the prevalence data in 247 patients with prostate cancer who were treated with conformal radiation therapy to a dose of 70 Gy. Grade 2 or higher rectal toxicities affected 4–5% of patients at 12–24 months after treatment and only 1.4% of patients at five years. Syndikus et al. [Citation12] found that the prevalence of moderate and severe GI toxicities lessened after three years. Like Abdalla [Citation3] and Karlsdottir [Citation4], Odrazka et al. [Citation22] found that the prevalence of rectal symptoms declined over time—from a maximum of 7.1% at 1.5 years to 2.5% at five years for grade 2 symptoms and from 1.9% at two years to 1.3% at five years for grade 3 symptoms. However, the prevalence curve in the Odrazka study clearly showed two peaks for both grade 2 and 3 toxicities. The first peak was found at approximately 1.5–2 years and the second lower peak at 4.5–5 years after treatment. Minimum prevalence was situated between these two peaks, at approximately three years from the end of RT. The Odrazka study of 320 patients and a median follow-up of 6.5 years, found the five-year risk of developing rectal toxicity grade 2 and grade 3 to be 15.6% and 7%, respectively, compared to 13.3% and 2.4% found in our study.
Time course of resolution
A similar number of patients with G2 + GI and GU toxicity (61% and 58%, respectively) had symptom resolution in a similar median length of time (3.2 vs. 3.0 years). This contrasts with the data of Zelefsky et al. [Citation1], where they found that it took 26 months for patients with a G2 + GI toxicity to resolve and only seven months for those with G2 + GU toxicity to resolve. They also found that more people with G2 + GI toxicity resolved than those with G2 + GU toxicity (91% vs. 81%).
Conclusion
Our study reports the temporal patterns of GI and GU toxicity post-NAHT and -RT. We show clearly that long-term toxicities continue to occur over many years. The percentage of patients suffering from G2 + toxicity at any time is however low. The resolution of these toxicities continues at a similar rate for the duration of the follow-up. These data may be used to advise patients about the frequency and projected time course of their expected GI and GU toxicities and may influence decision making regarding intervention versus conservative management.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2014.927072
Download PDF (99.4 KB)Acknowledgements
This research was supported by Ipsen pharmaceuticals, St. Luke's Institute of Cancer Research, Friends of St. Luke's, Cancer Research Ireland, and private donors. We thank Robert McNair for the database technical support.
Declaration of interest: Professor John Armstrong provided expert testimony on behalf of Amgen Inc. No other author has any conflict of interest.
References
- Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:1124–9.
- Christie D, Denham J, Steigler A, Lamb D, Turner S, Mameghan H, et al. Delayed rectal and urinary symptomatology in patients treated for prostate cancer by radiotherapy with or without short term neo-adjuvant androgen deprivation. Radiother Oncol 2005;77:117–25.
- Abdalla I, Ignacio L, Vaida F, Mei-Hsu, Awan A, Jani A, et al. Evolution of toxicity after conformal radiotherapy for prostate cancer. Prostate Cancer Prostatic Dis 2002;5: 296–303.
- Karlsdottir A, Muren LP, Wentzel-Larsen T, Dahl O. Late gastrointestinal morbidity after three-dimensional conformal radiation therapy for prostate cancer fades with time in contrast to genitourinary morbidity. Int J Radiat Oncol Biol Phys 2008;70:1478–86.
- Huang EH, Pollack A, Levy L, Starkschall G, Dong L, Rosen I, et al. Late rectal toxicity: Dose-volume effects of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2002;54:1314–21.
- Armstrong JG, Gillham CM, Dunne MT, Armstrong JG, Gillham CM, Dunne MT, et al. A randomized trial (Irish Clinical Oncology Research Group 97-01) comparing short versus protracted neoadjuvant hormonal therapy before radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2011;81:35–45.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–81.
- Cox DR. Regression models and life tables. J Res Stats Soc 1972;34:187–220.
- Fleming C, Kelly C, Thirion P, Fitzpatrick K, Armstrong J. A method for the prediction of late organ at risk toxicity after radiotherapy of the prostate using equivalent uniform dose. Int J Radiat Oncol Biol Phys 2011;80:608–13.
- Dearnaley DP, Sydes MR, Graham JD, Aird EG, Bottomley D, Cowan RA, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87.
- Syndikus I, Morgan RC, Sydes MR, Graham JD, Dearnaley DP. Late gastrointestinal toxicity after dose- escalated conformal radiotherapy for early prostate cancer: Results from the UK Medical Research Council RT01 trial (ISRCTN47772397). Int J Radiat Oncol Biol Phys 2010;77:773–83.
- Jereczek-Fossa BA, Zerini D, Fodor C, Santoro L, Serafini F, Cambria R, et al. Correlation between acute and late toxicity in 973 prostate cancer patients treated with three-dimensional conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:26–34.
- Peeters STH, Heemsbergen WD, Koper PC, van Putten WL, Slot A, Dielwart MF, et al. Dose response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter phase III randomized trial comparing 68 Gy with 78 Gy. J Clin Oncol 2006;24:1990–6.
- Al-Mamgani A, Van Putten WLJ, Heemsbergen WD, Van Leenders GJLH, Slot A, Dielwart MFH, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008;72:980–8.
- Peeters STH, Heemsbergen WD, van Putten WLJ, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: Results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 2005;61:1019–34.
- Nakamura RA, Monti CR, Castilho LN, Trevisan FA, Valim AC, Reinato JA. Prognostic factors for late urinary toxicity Grade 2–3 after conformal radiation therapy on patients with prostate cancer. Int Braz J Urol 2007;33:652–61.
- Scott CB, Pajak TF. LENT: A good beginning but.. Int J Radiat Oncol Biol Phys 1995;31:1347–8.
- Gravis G, Marino P, Joly F, Oudard S, Priou F, Esterni B, et al. Patients’ self-assessment versus investigators’ evaluation in a phase III trial in non-castrate metastatic prostate cancer (GETUG-AFU 15). Eur J Cancer 2014;50:953–62.
- Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, et al. Comparison of conventional-dose versus high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate. JAMA 2005;294:1233–9.
- Capp A, Inostroza-Ponta M, Bill D, Moscato P, Lai C, Christie D, et al. Is there more than one proctitis syndrome? A revisitation using data from the TROG 96.01 trial. Radiother Oncol 2009;90:400–7.
- Odrazka K, Dolezel M, Vanasek J, Vaculikova M, Zouhar M, Sefrova J, et al. Time course of late rectal toxicity after radiation therapy for prostate cancer. Prostate Cancer Prostatic Dis 2010;13:138–42.
- Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M.D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74.
- Dearnaley DP, Hall E, Lawrence D, Huddart RA, Eeles R, Nutting CM, et al. Phase III pilot study of dose escalation using conformal radiotherapy in prostate cancer: PSA control and side effects. Br J Cancer 2005; 92:488–98.