Abstract
Background. Vascular endothelial growth factor (VEGF-A) is a key regulator of tumor-induced angiogenesis and essential for tumor growth and distant tumor spread. The aim of the present study was to evaluate the role of VEGF-A polymorphisms and haplotypes for metastatic progression in breast cancer patients.
Material and methods. We performed a prospective study including 801 breast cancer patients. Occurrence of metastases was examined in regular follow-up investigations. Seven VEGF-A polymorphisms were selected and determined by 5′-nuclease assays (TaqMan). The selection of VEGF-A variants was based upon their location (promoter or UTR) as well as a minor allele frequency of at least 0.10. Haplotypes and linkage disequilibrium were determined using the Haploview program.
Results. Within a median follow-up time of 84 months, 165 (21%) patients developed distant metastases. In univariate analysis, carriers of the CCCCC haplotype formed by five polymorphisms upstream the coding region were at decreased risk of distant metastases [hazard ratio (HR) = 0.743; 95% CI 0.579–0.953; p = 0.019]. Univariate analysis also revealed a decreased risk of distant metastases for postmenopausal patients carrying the -634G> C polymorphism (HR 0.704; 95% CI 0.514–0.965; p = 0.029) and the CCCCC haplotype (HR = 0.645; 95% CI 0.464–0.898; p = 0.009). After adjustment for other co-variates, the HR for distant metastases was 0.651 (95% CI 0.447–0.948) for postmenopausal carriers of the -634G> C polymorphism (p = 0.025; corrected p-value = 0.262), and 0.586 (95% CI 0.393–0.873) for postmenopausal patients with the CCCCC haplotype (p = 0.009, corrected p-value = 0.189).
Conclusion. The results from univariate and multivariate analyses suggest an influence of VEGF-A gene variants on the development of distant metastases in breast cancer patients. However, none of the observed associations reached statistical significance after correction for the effects of multiple testing. Additional prospective and sufficiently powered studies are essential before firm conclusions about the role of VEGF-A gene variants for distant progression in breast cancer can be drawn.
Vascular endothelial growth factor (VEGF-A) plays a pivotal role in angiogenesis, a key event in the process of tumor growth and metastatic tumor spread. Activation of the VEGF/VEGF-receptor axis triggers multiple signaling networks that promote endothelial cell growth, migration, differentiation, and mobilization of endothelial progenitor cells from the bone marrow into the peripheral circulation [Citation1]. In addition, VEGF-A mediates vessel permeability leading to the deposition of proteins in the interstitium that facilitate angiogenesis [Citation2].
Hypoxia is a major inducer of VEGF-A gene transcription [Citation3]. Apart from hypoxia, VEGF-A expression is regulated by several growth factors, cytokines, and hormones [Citation1,Citation3]. Estradiol has been shown to be a potent regulator of VEGF-A expression. In premenopausal women, significantly higher VEGF-A levels as well as cyclic variations of VEGF-A during the menstrual cycle have been observed [Citation4–6].
The regulation of gene expression in human is a critical and highly coordinated process. Promoters are the best-characterized transcriptional regulatory sequences in complex genomes located immediately upstream of transcription start sites. Further regulatory elements can sometimes be found in the untranslated regions (UTR). The gene for VEGF-A is located on chromosome 6p21.1. Its coding region spans approximately 14 kilobases and consists of eight exons [Citation7]. Single nucleotide polymorphisms (SNPs) in the promoter, 5′-, and 3′-UTRs have been found to affect protein translation efficiency and circulating plasma concentrations as well as tumor tissue expression of VEGF-A and may thus be associated with cancer risk and progression [Citation8,Citation9].
Data from previous investigations have suggested that VEGF-A polymorphisms may contribute to cancer susceptibility [Citation10,Citation11]. However, in a previous study on the association between VEGF-A gene polymorphisms and breast cancer risk, we could not detect a significant influence on the occurrence of breast cancer [Citation12]. Furthermore, previously performed meta-analyses failed to demonstrate convincing associations between VEGF-A SNPs and breast cancer risk [Citation13,Citation14].
Overexpression of VEGF-A has also been associated with tumor progression and poor prognosis in several tumor entities, including colorectal carcinoma, gastric carcinoma, pancreatic carcinoma, prostate cancer, and lung cancer [Citation1,Citation15,Citation16]. In breast cancer patients, increased VEGF-A expression has recently been associated with significantly shorter recurrence-free survival [Citation17].
Due to the central role of VEGF-A in cancer progression, we hypothesized that VEGF-A gene polymorphisms may influence the risk of developing distant metastases. To evaluate the role of VEGF-A SNPs and haplotpyes for the development of distant metastases in breast cancer patients, we performed the present prospective study.
Material and methods
Subjects
The present study included female patients with histologically confirmed breast cancer from the Austrian “Tumor of breast tissue: Incidence, Genetics and Environmental Risk factors” (TIGER) study [Citation12]. From the 804 participants of TIGER, 801 were eligible for inclusion. Three patients had developed a second primary invasive cancer other than breast cancer and were therefore excluded from analysis as to consider only events which may reflect breast cancer metastasization.
All subjects were recruited between January 2000 and September 2004 from patients attending the Division of Oncology, Department of Internal Medicine and the Department of Therapeutic Radiology and Oncology, Medical University Graz, Austria. Follow-up investigations were performed in regular intervals (3 months intervals in years 1–3, 6 months intervals in years 4–5, and 12 months intervals in years 6–10 after diagnosis) and included physical examination and eliciting of symptoms, laboratory tests including CEA and carbohydrate antigen 15-3 (CA 15-3), radiological assessment (mammography and breast ultrasound, liver ultrasound or computed tomography and chest x-ray), and gynecological examination. In symptomatic patients, a bone scintigraphy was performed. A diagnosis of bone metastasis was defined as a positive finding by bone scintigraphy confirmed by a positive finding using another imaging technique, such as x-ray, computed tomography and magnetic resonance imaging. The scanning techniques or examinations did not change over time.
Patients with a follow-up time shorter than four months were excluded from analysis. Loss to follow-up rate was 4.3% after three years and 8.9% after five years, respectively.
The study complied with the Declaration of Helsinki and was performed according to the Austrian Gene Technology Act. The protocol has been approved by the Ethical Committee of the Medical University Graz. Written informed consent was obtained from all participating subjects.
The REporting recommendations for tumor MARKer prognostic studies (REMARK) were used to design the research and present the data in present study [Citation18].
Selection of VEGF-A polymorphisms
With the use of the public NCBI SNP database and available literature, we selected VEGF-A candidate polymorphisms with a minor allele frequency of at least 0.10 and location in the promoter region, coding region or untranslated region of the VEGF-A gene. Using this approach, seven candidate polymorphisms were chosen for further analysis: -2578C> A (rs699947), -2489C> T (rs1005230), -1498C> T (rs833061), -634G> C (rs2010963), -7C> T (rs25648), 936C> T (rs3025039) and 1612G> A (rs10434).
DNA isolation and genotyping assays
Upon study entry, each participant had donated a tube of EDTA- blood, which was stored at −20°C. Genomic DNA was isolated by standard procedures and stored at 4°C. VEGF-A genotypes were determined between November 2005 and August 2006 using 5’-nuclease assays (TaqMan). Reaction conditions as well as primers and probe sets were as described previously [Citation19].
Construction of haplotypes and statistical analysis
Haplotypes and linkage disequilibrium were determined using the Haploview program (version 2.05, http://www.broad.mit.edu/personal/jcbarret/haploview/). Assignment of individual haplotype pairs was performed by the PHASE version 2.1 software [Citation20].
Statistic analysis was done using SPSS 20.0 for Windows. Primary endpoint was metastases-free survival defined as the time from diagnosis to any distant metastases. The time to the development of distant metastases was calculated from diagnosis, patients with a follow-up time shorter than four months were excluded from analysis. Numeric values were analyzed by Student's t-test, proportions of groups were compared by χ2-test. Cox proportional hazards analysis was performed to calculate the hazard ratio (HR) and 95% confidence interval (CI) to evaluate the influence of genotypes on the risk of distant metastases, additionally, multivariate Cox regression analysis was performed to adjust for other potential predictors of patients’ outcome. Variables have been checked for collinearity with the statistical software we used (SPSS 20.0).
It can be assumed that the genetic effects of SNPs located in the non-coding sequence of the VEGF-A gene will function by increasing or decreasing VEGF-A expression. Additive genetic models impose a structure in which each additional copy of the variant allele increases the effect. VEGF-A genotypes have therefore been coded assuming an additive model for all analyses (wildtype = 0; heterozygous carrier of minor allele or haplotype = 1, homozygous carrier = 2). The relationship between genotypes and distant metastases-free survival was analyzed by calculating cumulative survival rates by the Kaplan-Meier method and evaluating them by the log- rank test. Threshold for significance was p < 0.05. A Benjamini and Hochberg false discovery rate (FDR) control was made to adjust for the effects of multiple testing [Citation21].
Results
Patient and tumor characteristics and their association with the development of distant metastases are presented in . Median follow-up time was 84 months (95% CI 82–86 months). During follow-up, distant metastases (bone, n = 62; liver, n = 45; lung, n = 37; non-regional lymph nodes, n = 38; skin, n = 13; brain, n = 5, other sites, n = 6) were detected in 165 patients (21%), respectively. Genotypes did not deviate from Hardy-Weinberg equilibrium.
Table I. Baseline patient und tumor characteristics and the association with the development of distant metastases.
Haplotype analysis showed two separate blocks of high-linkage disequilibrium, formed by five polymorphisms (-2578C> A, -2489C> T, -1498C> T, -634G> C, -7C> T) upstream of the coding sequence (CCCCC, ATTGC, CCCGC, ATTGT) and two polymorphisms (936C> T, 1612G> A) downstream of the coding sequence (CA, CG, TG), respectively.
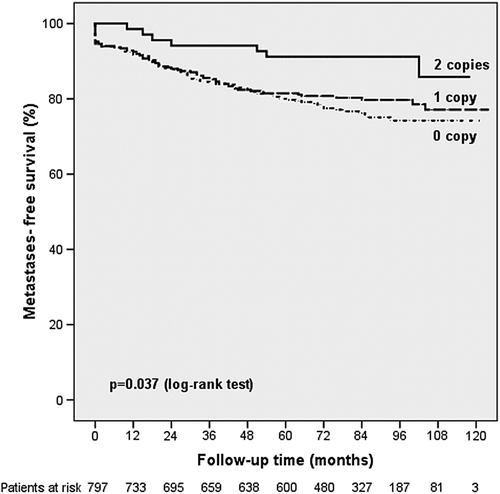
In Kaplan-Meier analysis, the VEGF-A CCCCC haplotype formed by the -2578C, -2489C, -1498C, -634C, and -7C alleles was significantly associated with increased distant metastases-free survival (p = 0.037; ). Univariate analysis revealed that the VEGF-A CCCCC haplotype was associated with a decreased risk of developing distant metastases (HR = 0.743; 95% CI 0.579–0.953; p = 0.019, ). None of the remaining genotypes or haplotypes was significantly associated with the occurrence of distant metastases. In a multivariate Cox regression model including age at diagnosis, tumor size, lymph node involvement, tumor grade, and hormonal receptor status as potential confounders, the hazard ratio for the development of distant metastases was 0.757 for carriers of the CCCCC haplotype (95% CI 0.571–1.003; p = 0.052).
Table II. Univariate and multivariate analysis of the association between VEGF-A gene variants and distant metastases.
In postmenopausal patients, univariate analysis showed a significant association between the VEGF-A -634G> C polymorphism and a decreased risk of distant metastases (HR = 0.704, 95% CI 0.514–0.965; p = 0.029, and ). After adjustment for other co-variates in multivariate analysis, the HR was 0.651 among carriers of the VEGF-A -634G> C polymorphism (95% CI 0.447–0.948; p = 0.025, corrected p-value = 0.262).
Table III. Univariate and multivariate analysis of the association between VEGF-A gene variants and distant metastases in postmenopausal breast cancer patients.
Table IV. Multivariate analysis of the association of the VEGF-A -634G> C polymorphisms and all co-variates included in the Cox regression analysis with metastases-free survival in postmenopausal patients.
Furthermore, univariate analysis showed a decreased risk of distant metastases among postmenopausal breast cancer patients carrying the CCCCC haplotype (HR = 0.645, 95% CI 0.464–0.898, p = 0.009, ). Kaplan-Meier analysis revealed a significantly increased metastases-free survival for postmenopausal carriers of the CCCCC haplotype (p = 0.024, ).
Figure 2. Association of the VEGF-A CCCCC haplotype with the development of distant metastases in postmenopausal patients.
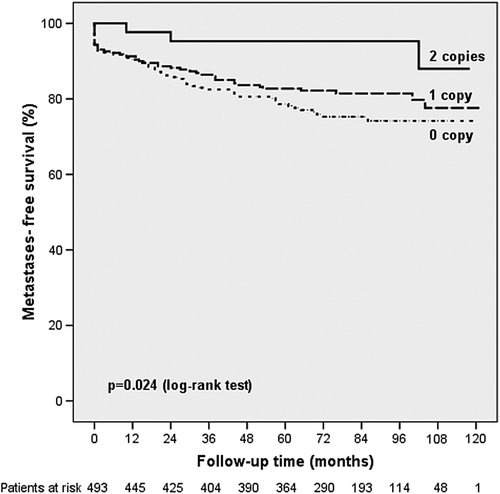
After adjustment of co-variates in multivariate analysis, the HR was 0.586 for the development of distant metastases among postmenopausal carriers of the CCCCC haplotype (95% CI 0.393–0.873; p = 0.009, corrected p-value = 0.189; and ).
Table V. Multivariate analysis of the association of the VEGF-A CCCCC haplotype and all co-variates included in the Cox regression analysis with metastases-free survival in postmenopausal patients.
The adjusted HR, CI, p-value for all co-variates included in the Cox regression analyses are presented in and . With the statistical software we used (SPSS 20.0), collinearities between the co-variates were not detected.
In 73% of postmenopausal patients, information on Her2 status was available. After inclusion of Her2 status in multivariate analysis in these patients, the HR for the development of distant metastases was 0.537 (95% CI 0.342–0.853; p = 0.006, corrected p-value = 0.252) for carriers of the CCCCC haplotype and 0.597 (95% CI 0.393–0.907; p = 0.016, corrected p-value = 0.224) for carriers of the VEGF-A -634G> C polymorphism.
Discussion
The present study was performed to investigate the influence of VEGF-A polymorphisms and haplotypes on the development of distant metastases in breast cancer patients. In univariate analysis, we found a significant influence of the CCCCC haplotype on the risk of developing distant metastases. Although a trend toward improved metastases-free survival was observed, this result did not reach significance in multivariate analysis. In postmenopausal breast cancer patients, univariate and multivariate analyses revealed an influence of the -634G> C polymorphism and the CCCCC haplotype on the development of distant metastases. However, after correction for the effects of multiple testing, none of the results retained statistical significance.
In view of the critical role of VEGF-A in tumor-related angiogenesis, the association of VEGF-A gene polymorphisms with cancer risk or prognosis has previously been investigated in various cancer entities. In colorectal cancer patients, Kim and co-workers demonstrated increased survival rates for carriers of the VEGF-A -634G/C or C/C genotype compared to patients with the G/G genotype [Citation22]. Similarly, Heist and colleagues found that early-stage non-small cell lung cancer patients carrying the variant C allele of the VEGF-A -634G> C polymorphism had a significantly improved time to recurrence [Citation23]. In esophageal cancer, the CGC haplotype formed by the VEGF-A -1498C, -634G, and the 936C alleles was associated with reduced overall survival [Citation24].
In a large study by Lu and colleagues who evaluated the effects of three VEGF-A gene polymorphisms (-1498T> C, -634G> C, and 936C> T) on survival in 1193 Chinese breast cancer patients, carriage of the -1498C and -634G allele was significantly associated with decreased survival. These findings compare well with the data from our study that show an increased metastases-free survival for postmenopausal breast cancer patients carrying the -634C allele and the CCCCC haplotype that is containing the -634C allele [Citation25].
In contrast, Hefler and colleagues reported that simultaneous carriage of the homozygous VEGF-A -2578C/C, -1154G/G, -634C/C genotypes was associated with a shortened overall survival in 563 patients with ovarian cancer [Citation26]. In a study by Luo et al., the -634CC genotype was significantly associated with large tumor size and high tumor grade but not related with other tumor characteristics, such as regional or distant metastasis [Citation27]. Maae and colleagues reported an inferior prognosis for patients with HER2-positive breast cancer [Citation28]. Furthermore, Sa-Nguanraksa et al. showed an association of VEGF-A 634G/C polymorphism with perineural invasion and advanced tumor stage and observed a lower diseasefree survival among individuals with the VEGF-A634C/C genotype [Citation29].
The -634 G> C polymorphism has been found to lie within a myeloid zinc finger protein (MZF1) binding site [Citation30], and its function currently discussed controversially. In some studies, higher VEGF-A levels or no association with the -634C/C genotype have been found [Citation12,Citation29,Citation31]. Awata et al. reported that individuals with the -634 C/C genotype had a higher fasting serum VEGF-A level than those with other genotypes, Sa-Nguanraksa et al. showed that patients with the VEGF-A -634C/C genotype exhibited the highest VEGF-A mRNA levels [Citation29,Citation31].
In a previous study, we were unable to detect any clear effects of VEGF-A genotypes on VEGF-A plasma levels [Citation12]. This is in line with a previously published genome-wide association study and with a publication from Berrahmoune and co-workers, who reported that plasma VEGF-A concentrations were under strong genetic control in healthy families, but not influenced by VEGF-A genotypes at positions -1498, -634 or 936 [Citation32,Citation33].
In contrast, Watson et al. showed a lower lipopolysaccharide-stimulated VEGF-A production by peripheral blood mononuclear cells for the -634C allele compared to the -634G allele suggesting that the presence of the C allele may disrupt the MZF1 transcription factor binding site [Citation30]. In an in vitro study by Stevens and colleagues, carriage of a haplotype containing the -1498C/-634G polymorphisms was found to significantly increase basal VEGF-A promoter activity and phorbol ester- induced responsiveness compared with the presence of a haplotype containing the + 1498T/-634C polymorphisms [Citation34]. However, attributing functional causality to these findings should be limited, as there is still debate regarding the true function of VEGF-A polymorphisms.
In premenopausal women, significantly higher VEGF-A levels with estrogen as a potent regulator of VEGF-A expression have been shown [Citation4,Citation5]. The effect of estrogen on VEGF-A expression is predominately mediated by estrogen-receptor responsive elements in the VEGF-A promoter, furthermore, co-binding of the activated estrogen receptor with cMyc, BRCA1 and other transcription factors has been found to contribute to the regulation of VEGF-A gene transcription [Citation6]. Differences in the estrogenic influence on VEGF-A gene expression might serve to explain the discrepancy of our findings between pre- and postmenopausal women.
Major strengths of the present study include the relatively large number of incident cases from an ethnic homogenous study population as well as the prospective study design with a median follow-up time of 84 months. Furthermore, both patient recruitment and clinical outcome data collection were carried out independently without knowledge of polymorphism status. Additionally, high quality genotyping of polymorphisms that have been selected because of their functionality and the analyses of haplotypes are provided.
In a previous analysis on the same patient cohort, we investigated the association of the selected VEGF-A genotypes with breast cancer risk and baseline characteristics such as tumor stage, lymph node involvement, tumor grade, and hormonal receptor status. As one of the main results, an association between the VEGF-A -634C allele and small tumor size has been observed. These data have been published previously [Citation12]. In the current analysis, carriership of the -634C allele has been coupled to an increased metastasis-free survival. However, the absence of collinearities and the results of the multivariate analyses suggest an independent association between VEGF-A genotypes and metastasis-free survival.
One of the major problems of genetic association studies is the increasing risk of type 1 error (false positive) findings after multiple testing. After correction for the effects of multiple testing, none of the results obtained in the present investigation has reached a p-value ≤ 0.05. Applying statistical corrections for multiple testing can be used to reduce this type 1 error risk, but at the same time, may increase type 2 error risk (false negative findings). To reduce the risk of both false positive and false negative findings, additional prospective and well-powered studies are needed.
Due to its central role in angiogenesis and the pathogenesis of human cancers, the VEGF-A mediated pathway has been a major focus of basic research and drug development that has led to the development of anti-cancer agents that selectively target this pathway. In view of the potential role of VEGF-A polymorphisms for VEGF-A activity, their role for the pharmacogenetics of VEGF-A inhibitors has been investigated in recent clinical studies and preliminary data show that VEGF-A SNPs may be predictive for the efficacy and toxicity of anti-VEGF-A agents [Citation35,Citation36].
Conclusion
In the present investigation, results from univariate and multivariate analyses suggest an association between VEGF-A gene variants and distant metastases in breast cancer patients that did not retain statistical significance after correction for the effects of multiple testing. Additional prospective and sufficiently powered studies are needed before firm conclusions about the role of VEGF-A gene variants for distant progression in breast cancer can be drawn.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the Anniversary Fund of the Österreichische Nationalbank (Project Nr. 10609).
References
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27.
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol 1995;146:1029–39.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76.
- Hyder SM, Nawaz Z, Chiappetta C, Stancel GM. Identification of functional estrogen response elements in the gene coding for the potent angiogenic factor vascular endothelial growth factor. Cancer Res 2000;60:3183–90.
- Dabrosin C. Variability of vascular endothelial growth factor in normal human breast tissue in vivo during the menstrual cycle. J Clin Endocrinol Metab 2003;88:2695–8.
- Dadiani M, Seger D, Kreizman T, Badikhi D, Margalit R, Eilam R, et al. Estrogen regulation of vascularendothelial growth factor in breast cancer in vitro and in vivo: The role of estrogen receptor alpha and c-Myc. Endocr Relat Cancer 2009;16:819–34.
- Brogan IJ, Khan N, Isaac K, Hutchinson JA, Pravica V, Hutchinson IV. Novel polymorphisms in the promoter and 5′ UTR regions of the human vascular endothelial growth factor gene. Hum Immunol 1999;60:1245–9.
- Renner W, Kotschan S, Hoffmann C, Obermayer-Pietsch B, Pilger E. A common 936 C/T mutation in the gene for vascular endothelial growth factor is associated with vascular endothelial growth factor plasma levels. J Vasc Res 2000; 37:443–8.
- Koukourakis MI, Papazoglou D, Giatromanolaki A, Bougioukas G, Maltezos E, Sivridis E. VEGF gene sequence variation defines VEGF gene expression status and angiogenic activity in non-small cell lung cancer. Lung Cancer 2004;46:293–8.
- Rodrigues P, Furriol J, Tormo E, Ballester S, Lluch A, Eroles P. The single-nucleotide polymorphisms + 936 C/T VEGF and −710 C/T VEGFR1 are associated with breast cancer protection in a Spanish population. Breast Cancer Res Treat 2012;133:769–78.
- Hansen TF, Jakobsen A. Clinical implications of genetic variations in the VEGF system in relation to colorectal cancer. Pharmacogenomics 2011;12:1681–93.
- Langsenlehner U, Wolf G, Langsenlehner T, Gerger A, Hofmann G, Clar H, et al. Genetic polymorphisms in the vascular endothelial growth factor gene and breast cancer risk. The Austrian “tumor of breast tissue: Incidence, genetics, and environmental risk factors” study. Breast Cancer Res Treat 2008;109:297–304.
- Jain L, Vargo CA, Danesi R, Sissung TM, Price DK, Venzon D, et al. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol Cancer Ther 2009;8:2496–508.
- Liu L, Liu L, Zeng F, Wang K, Huang J, Xin L, et al. Meta-analysis of the association between VEGF-634 G & gt; C and risk of malignancy based on 23 case-control studies.J Cancer Res Clin Oncol 2011;137:1027–36.
- Lee JC, Chow NH, Wang ST, Huang SM. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 2000;36:748–53.
- Berns EM, Klijn JG, Look MP, Grebenchtchikov N, Vossen R, Peters H, et al. Combined vascular endothelial growth factor and TP53 status predicts poor response to tamoxifen therapy in estrogen receptor-positive advanced breast cancer. Clin Cancer Res 2003;9:1253–8.
- Maae E, Olsen DA, Steffensen KD, Jakobsen EH, Brandslund I, Sørensen FB, et al. Prognostic impact of placenta growth factor and vascular endothelial growth factor A in patients with breast cancer. Breast Cancer Res Treat 2012;133:257–65.
- McShane L, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM for the Statistics Subcommittee of the NCI-EORTC Working Group on Cancer diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Eur J Cancer 2005;41:1690–6.
- Langsenlehner U, Krippl P, Renner W, Yazdani-Biuki B, Eder T, Koppel H, et al. Interleukin-10 promoter polymorphism is associated with decreased breast cancer risk. Breast Cancer Res Treat 2005;90:113–5.
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 2001;68:978–89.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc Series B 1995;57:289–300.
- Kim JG, Chae YS, Sohn SK, Cho YY, Moon JH, Park JY, et al. Vascular endothelial growth factor gene polymorphisms associated with prognosis for patients with colorectal cancer. Clin Cancer Res 2008;14:62–6.
- Heist RS, Zhai R, Liu G, Zhou W, Lin X, Su L, et al. VEGF polymorphisms and survival in early-stage non-small-cell lung cancer. J Clin Oncol 2008;26:856–62.
- Bradbury PA, Zhai R, Ma C, Xu W, Hopkins J, Kulke MJ, et al. Vascular endothelial growth factor polymorphisms and esophageal cancer prognosis. Clin Cancer Res 2009;15: 4680–5.
- Lu H, Shu XO, Cui Y, Kataoka N, Wen W, Cai Q, et al. Association of genetic polymorphisms in the VEGF gene with breast cancer survival. Cancer Res 2005;65: 5015–9.
- Hefler LA, Mustea A, Könsgen D, Concin N, Tanner B, Strick R, et al. Vascular endothelial growth factor gene polymorphisms are associated with prognosis in ovarian cancer. Clin Cancer Res 2007;13:898–901.
- Luo T, Chen L, He P, Hu QC, Zhong XR, Sun Y, et al. Vascular endothelial growth factor (VEGF) gene polymorphisms and breast cancer risk in a Chinese population. Asian Pac J Cancer Prev 2013;14:2433–7.
- Maae E, Andersen RF, Steffensen KD, Jakobsen EH, Brandslund I, Sørensen FB, et al. Prognostic impact of VEGFA germline polymorphisms in patients with HER2-positive primary breast cancer. Anticancer Res 2012;32: 3619–27.
- Sa-Nguanraksa D, Chuangsuwanich T, Pongpruttipan T, Kummalue T, Rojananin S, Ratanawichhitrasin A, et al. Vascular endothelial growth factor 634G/C polymorphism is associated with increased breast cancer risk and aggressiveness. Mol Med Rep 2013;8:1242–50.
- Watson CJ, Webb NJ, Bottomley MJ, Brenchley PE. Identification of polymorphisms within the vascular endothelial growth factor (VEGF) gene: Correlation with variation in VEGF protein production. Cytokine 2000;12: 1232–5.
- Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A common polymorphism in the 5’-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes 2002; 51:1635–9.
- Debette S, Visvikis-Siest S, Chen MH, Ndiaye NC, Song C, Destefano A, et al. Identification of cis- and trans-acting genetic variants explaining up to half the variation in circulating vascular endothelial growth factor levels. Circ Res 2011;109:554–63.
- Berrahmoune H, Herbeth B, Lamont JV, Masson C, Fitzgerald PS, Visvikis Siest S. Heritability for plasma VEGF concentration in the Stanislas family study. Ann Hum Genet 2007;71:54–63.
- Stevens A, Soden J, Brenchley PE, Ralph S, Ray DW. Haplotype analysis of the polymorphic human vascular endothelial growth factor gene promoter. Cancer Res 2003; 63:812–6.
- Formica V, Palmirotta R, Del Monte G, Savonarola A, Ludovici G, De Marchis ML, et al. Predictive value of VEGF gene polymorphisms for metastatic colorectal cancer patients receiving first-line treatment including fluorouracil, irinotecan, and bevacizumab. Int J Colorectal Dis 2011; 26:143–51.
- Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol 2008;26:4672–8.