Abstract
Background. Many cancer patients receive chemotherapy and radiotherapy their last 30 days [end of life (EOL)]. The benefit is questionable and side effects are common. The aim of this study was to investigate what characterized the patients who received chemo- and radiotherapy during EOL, knowledge that might be used to improve practice.
Methods. Patients dead from cancer in 2005 and 2009 were analyzed. Data were collected from hospital medical records. When performance status (PS) was not stated, PS was estimated from other information in the records. A Glasgow Prognostic Score (GPS) of 0, 1 or 2 was assessed from blood values (CRP and albumin). A higher score is associated with a shorter prognosis.
Results. In total 616 patients died in 2005; 599 in 2009. Among the 723 analyzed, median age was 71; 42% had metastases at diagnosis (synchronous metastases); 53% had PS 2 and 16% PS 3–4 at the start of last cancer therapy. GPS at the start of last cancer therapy was assessable in 70%; of these, 26% had GPS 1 and 35% GPS 2. Overall, 10% received chemotherapy and 8% radiotherapy during EOL. The proportions varied significantly between the different types of cancer. Multivariate analyses revealed that those at age < 70 years, GPS 2, no contact with our Palliative Care Unit and synchronous metastases received most chemotherapy the last 30 days. PS 3–4, GPS 2 and synchronous metastases were strongest associated with radiotherapy the last 30 days.
Conclusion. Ten percent received chemotherapy and 8% radiotherapy the last 30 days of life. GPS 2 and synchronous metastases were most significantly associated with cancer therapy the last 30 days of life, indicating that in general, patients with the shortest survival time after diagnosis of cancer received more chemo- and radiotherapy during EOL than other patients.
Chemotherapy remains one of the most common palliative therapies for patients with advanced cancer. The aim is to achieve temporary disease control, relieve symptoms and prolong survival. However, not all patients respond to therapy, the survival benefit is often limited and side effects are frequent. Thus, it is challenging to balance expected benefits of therapy with potential disadvantages.
Studies have demonstrated that 13–43% of advanced cancer patients receive chemotherapy during the last 30 days of life [Citation1–6], and that the proportion has increased over time [Citation7–10]. Chemotherapy during end of life (EOL) consumes valuable time, and might have a negative impact on quality of life (QoL) and survival. In a study of patients with advanced non-small cell lung cancer, those randomized to early palliative care, in addition to standard oncological care, received less chemotherapy during EOL, had better QoL and longer survival than those receiving standard oncological care alone [Citation4]. Furthermore, chemotherapy during EOL might increase the risk of hospitalization and dying in hospital [Citation4,Citation10].
There are fewer studies of palliative radiotherapy during EOL, possibly since the main intention is to relieve symptoms; and there are fewer concerns about side effects. In two studies, 8–19% of patients received radiotherapy the last 30 days of life [Citation11,Citation12]. The benefit of radiotherapy during EOL is, however, questionable; in a third study, only 58% completed treatment as planned, 26% had symptom relief whereas symptoms progressed in 52% [Citation13].
The reasons why many patients receive chemo- or radiotherapy near death are unclear. One reason might be that it is difficult for physicians to estimate survival time, or that they are overly optimistic [Citation14,Citation15]. Prognostic scales might be of help. WHO/ECOG performance status (PS) is the most extensively studied and one of the strongest prognostic factors in cancer patients [Citation16]. The Glasgow Prognostic Score (GPS) is based on CRP- and albumin-values, and is a significant prognostic factor in cancer patients [Citation17]. Another possible reason for administering therapy during EOL might be that physicians pay too little attention to the fact that many cancer therapies are introduced after clinical trials on patients who in general are younger, have better PS and less comorbidity than many patients seen in the clinic. Thus, the average patient might have less of a chance to tolerate and respond to the therapy than those enrolled in trials [Citation18,Citation19]. Some physicians might also be reluctant to tell their patients that they are likely to be approaching the EOL, especially if the patients want more treatment [Citation19].
The overall aim of our study was to investigate what characterized patients receiving chemo- and radiotherapy during their last 30 days of life.
Material and methods
Design and approval
This retrospective study was approved by the Regional Committee for Medical and Health Research Ethics in Central Norway.
Patients
The Norwegian Cause of Death Registry provided a list of all patients who died from cancer in Sør-Trøndelag county, Norway (301 000 inhabitants), in 2005 and 2009. Patients were eligible for this study if there was information about cancer diagnosis in the medical records at our hospital; if the patients received at least one cancer therapy; if the last cancer therapy was considered palliative; and if the malignancy was non-hematological.
The last year for which the registry had complete data when the study was initiated was 2009; 2005 was chosen to investigate whether the use of therapy during EOL had changed over time. Data were collected from the hospital medical records by MA and MAG. When PS was not stated, PS was estimated from other information in the patients’ records. We recorded whether patients had metastases at diagnosis (synchronous metastases) since survival-time from diagnosis and extent of disease has been associated with cancer therapy during EOL [Citation8,Citation12,Citation20].
Glasgow Prognostic Score (GPS)
Patients are given a score of 0, 1 or 2 depending on CRP and albumin. A high score is associated with a poor prognosis [Citation17]. CRP > 10 mg/l and albumin < 35 g/l scores 2; CRP > 10 mg/L scores 1; CRP ≤ 10 mg/L and albumin < 35 g/l scores 0.
Statistical considerations
Group comparisons were conducted using the χ2-tests. Survival was estimated using the Kaplain-Meier method. Cox's proportional hazard method was used in the multivariate survival analyses. Binary logistic regression was used in the multivariate analyses of which factors were most significantly associated with chemo- or radiotherapy during EOL; all significant prognostic factors in the univariate analyses were entered in the models (except cancer type since the number of patients enrolled were very small for many types of cancer). Significance level was defined as p < 0.05.
Results
Patient characteristics
In total 615 patients died of cancer in 2005 and 599 in 2009; 723 were analyzed. Reasons for exclusion were: no information about cancer in the medical records (n = 101); curative intention of the last cancer therapy (n = 85); hematological malignancies (n = 51); and no cancer therapy (n = 254). Reasons for no cancer therapy were poor PS (34%); no therapy available (20%); comorbidity (18%); dementia (9%); and patients’ wish (7%).
Median age was 71 years (range: 6–99), 3 patients were < 18 years, and 51% men. Twenty-one percent had lung cancer (of those, 26% had small cell lung cancer), 15% colorectal, 13% prostate and 9% breast cancer () and 42% had metastases when diagnosed with cancer (synchronous metastases).
Table I. Chemo- and radiotherapy the last 30 and 14 days, total number of chemotherapy regimens and courses of radiotherapy administered from diagnosis until death for the different types of cancer (n > 10).
Chemo- and radiotherapy from diagnosis until death
Mean number of chemotherapy regimens for the entire cohort from diagnosis was 0.98 (range 0–9); 32% received one regimen, 18% two regimens and 8% ≥ three regimens. Mean number of courses of radiotherapy was 0.85 (range 0–7); 37% received one course, 11% two courses and 7% ≥ three courses ().
Chemotherapy was the last cancer therapy in 39%, radiotherapy in 31%, surgery in 11% and hormonal therapy in 19%, and 36% received cancer therapy the last 30 days and 25% the last 14 days of life.
Chemo- and radiotherapy during the last 30 days of life
There were no gender-differences in the use of chemotherapy (men: 11%, women: 10%; p = 0.90) or radiotherapy (men: 8%, women: 9%; p = 0.73) the last 30 days (); nor were there differences between 2005 and 2009 (chemotherapy: 11% vs. 10%; p = 0.95; radiotherapy: 10% vs. 7%; p = 0.17). Consequently, all patients were analyzed as one cohort.
Table II. Characteristics of patients receiving no chemo- or radiotherapy during end of life (EOL), chemotherapy during EOL and radiotherapy during EOL. Five patients received both chemo- and radiotherapy during EOL. The p-values are for comparisons with the group who did not receive any chemo- or radiotherapy during EOL.
Ten percent (n = 75) received chemotherapy and 8% (n = 61) had radiotherapy their last 30 days; 5% (n = 36) received chemotherapy and 4% (n = 29) had radiotherapy the last 14 days. Five patients received both chemotherapy and radiotherapy the last 30 days.
When chemotherapy was the last treatment, the median time from start until death was 4.2 months; from end of treatment 2.2 months. Five percent (n = 38) started chemotherapy within 30 days of death. When radiotherapy was the last treatment, the median time from start until death was 2.4 months; from end of radiotherapy 2.1 months. Seven percent (n = 48) started radiotherapy within 30 days.
The highest proportion receiving chemotherapy the last 30 days was observed for patients with pancreatic (31%), lung (19%), melanoma (15%) and breast cancer (12%). The highest proportion receiving radiotherapy was observed in lung (16%), breast (8%), CNS-tumors (7%) and colorectal cancer (7%) (). Those with synchronous metastases received more chemotherapy (15% vs. 8%; p = 0.024) and radiotherapy (13% vs. 8%; p = 0.005) the last 30 days than other patients.
The indications for radiotherapy the last 30 days were painful bone metastases (33%); compression of central airways (16%) or the spinal cord (11%) and brain metastases (11%). In total 33% received 1–2 fractions; 31% 3–5 fractions; 25% 6–10 fractions; and 11% > 10 fractions and 82% completed radiotherapy as planned ().
Table III. Sixty-one patients (8%) received radiotherapy the last 30 days of life. Indications and fractionation schedules, completion rate, and reasons for discontinuation of radiotherapy are listed here.
Age and contact with our hospital Palliative Care Unit (PCU)
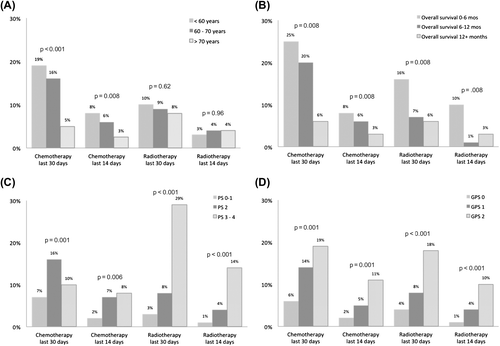
Patients < 70 years received more chemotherapy than older patients (p = 0.008), but not more radiotherapy (p = 0.62) the last 30 days ().
Forty-nine percent of patients were referred to our PCU (the only PCU in our region). Median time from the first contact with the PCU until death was 1.7 months; in 33% the first contact was within one month, and in 19% within two weeks of death.
Patients referred to the PCU were younger (median age: 69.5 vs. 74; p < 0.001), had more synchronous metastases (39% vs. 30%; p = 0.039), similar survival (18.5 vs. 15.5 months; p = 0.78), received similar amount of radiotherapy (7% vs. 10%; p = 0.11), but less chemotherapy (8% vs. 13%; p = 0.033) during EOL.
Associations between Performance status, Glasgow Prognostic Score, overall survival time and chemo- and radiotherapy during end of life
PS at the start of the last cancer therapy was stated in 47% and was estimated in 49%. 47% had PS 0–1, 34% PS 2 and 16% PS 3–4. Both stated and estimated PS were significant prognostic factors (p < 0.001). Median overall survival times were (all combined): PS 0: 8.9 months; PS 1: 5.1 months; PS 2: 2.8 months; PS 3: 1.6 months; and PS 4: 0.4 months.
Among those receiving chemotherapy the last 30 days (n = 75), 32% had PS 0–1, 53% had PS 2 and 16% PS 3–4. The proportion was higher for PS 2 (16%) and PS 3–4 (10%) than for PS 0–1 (7%) patients (p < 0.001). Among those who received radiotherapy the last 30 days (n = 61), 15% had PS 0–1, 31% had PS 2 and 54% PS 3–4. The proportion was higher for PS 2 (7%) and PS 3–4 (8%) than for PS 0–1 (2%) patients (p = 0.006) ().
GPS at the start of last cancer therapy was assessable in 509/723 patients (70%), and was a significant prognostic factor: GPS 0 (n = 201): 5.0 months, GPS 1 (n = 130): 3.1 months and GPS 2 (n = 178): 1.9 months (p < 0.001). Both GPS score (p < 0.001) and PS (p < 0.001) remained significant in the multivariate regression survival analysis.
The proportions of patients receiving chemo- or radiotherapy their last 30 or 14 days were highest among those with a GPS 2 (). Patients who lived less than 12 months from diagnosis received more chemotherapy (p = 0.008) the last 30 days; those who lived less than six months had more radiotherapy the last 30 days (p < 0.001) ().
Survival
Median overall survival from diagnosis for all patients was 16.8 months; no chemo- or radiotherapy during EOL: 19.4 months; chemotherapy administered during EOL: 7.3 months; radiotherapy administered during EOL: 8.5 months.
Multivariate analyses and comparisons with those who received no chemo- or radiotherapy during EOL
Age < 70 years (p < 0.001), GPS 2 (p = 0.009), no contact with the PCU (p = 0.006) and synchronous metastases (p = 0.32) remained significantly associated with receiving chemotherapy the last 30 days in the multivariate analysis. PS 3–4 (p < 0.001), GPS 2 (p = 0.005) and synchronous metastases (p = 0.005) remained significantly associated with receiving radiotherapy the last 30 days ().
Table IV. Multivariate analyses of factors associated with (A) chemotherapy and (B) radiotherapy during the last 30 days of life. Characteristics statistically significantly associated with chemo- or radiotherapy during EOL in the univariate analyses were entered in the models.
Characteristics of patients who received no chemo- or radiotherapy during EOL, those who received chemotherapy during EOL and those who received radiotherapy during EOL are listed in . In addition to the factors identified in the multivariate analyses, patients receiving chemotherapy during EOL had a poorer PS (68% vs. 44% PS 2–4; p < 0.001), received a higher mean number of chemotherapy regimens in total (1.75 vs. 0.90 regimens; p < 0.001), had a shorter median survival time from diagnosis until death (7.3 vs. 19.5 months; p = 0.003) and from metastases until death (4.7 vs. 11.7 months; p = 0.001) than those who did not receive chemotherapy or radiotherapy during EOL. Those who received radiotherapy during EOL received more courses of radiotherapy in total (mean 1.66 vs. 0.79; p < 0.001), had a shorter survival time from diagnosis until death (8.5 vs. 19.5 months; p = 0.007) and from metastases until death (7.6 vs. 11.7 months; p = 0.029) than those who did not receive chemotherapy or radiotherapy during EOL.
Hospital admissions and death
Patients receiving chemotherapy (88% vs. 65%; p < 0.001) or radiotherapy the last 30 days (93% vs. 65%; p < 0.001) had more hospital admissions the last 30 days.
In total 43% (n = 310) died in hospital, 17% (n = 126) at nursing homes and 8% (n = 56) at home; 32% died outside of hospital at an unknown location. Those receiving chemotherapy (75% vs. 39%; p < 0.001) or radiotherapy the last 30 days (53% vs. 42%; p = 0.017) more often died in hospital.
Twenty-nine patients (4%) died from complications of cancer therapy: postoperative complications (n = 12); neutropenic infections (n = 10); hemorrhagia (n = 4); and other (n = 3).
Discussion
In our cohort, 10% received chemotherapy and 8% radiotherapy the last 30 days of life. Age < 70 years, GPS 2, no contact with our PCU and synchronous metastases were most strongly associated with chemotherapy; PS 3–4, GPS 2 and synchronous metastases were most strongly associated with radiotherapy the last 30 days. These characteristics represented the main differences between those who received chemo- or radiotherapy during EOL, and those who did not. There were significant differences in the use of late chemo- and radiotherapy depending on type of cancer, but not between the years 2005 and 2009.
There was no clear association between use of late chemotherapy and chemosensitivity. Most chemotherapy during EOL was administered for pancreatic and non-small cell lung cancer, which are considered medium chemosensitive diseases. Furthermore, in 8% of the cases, patients had received more than two previous regimens, though there are limited data on the effect of chemotherapy beyond second-line for several solid tumors. Patients receiving chemo- or radiotherapy the last 30 days were more likely to be admitted to hospital during EOL and to die in hospital.
The use of chemotherapy during EOL in our cohort (10%) was lower than reported in other studies (13–43%) [Citation1–6,Citation21]. Considering that 256 (21%) of the 1214 cancer-patients who died in our county in 2005 and 2009 never received any chemotherapy, it was actually lower at 8%. However, studies of chemotherapy during EOL are not necessarily comparable. They have been conducted in different countries at different times; and there appears to be variations in patient selection, healthcare systems, economic incentives and aggressiveness in cancer care [Citation1–6,Citation21]. Possible explanations for less use of chemotherapy during EOL in our population might be that the healthcare system in Norway is public; and there are no economic incentives influencing the use of chemo- or radiotherapy [Citation11,Citation22].
The use of radiotherapy during EOL in our cohort was similar to other reports. In a large registry-based study 7.6% received radiotherapy the last 30 days (8% in our study) and the likelihood of receiving radiotherapy was highest for lung cancer patients [Citation11]. In another study, 19% of patients with incurable non-small cell lung cancer received radiotherapy their last 30 days (16% in our study) [Citation12].
There is limited data on the effect of late radiotherapy. Considering that the median time to pain relief after irradiation of bone metastases is three weeks [Citation23], it is questionable whether patients who receive radiotherapy within 30 days of death experience any clinically relevant benefit. In a study of 33 patients who died within 30 days of start of radiotherapy, 26% had symptom relief whereas 52% experienced symptom progression. Only 58% completed radiotherapy as planned [Citation13], compared with 82% in our study; suggesting that hypo-fractionated radiotherapy should be used more often in patients with advanced cancer and a short life expectancy [Citation13].
Similar to results of other studies, overall survival among patients receiving chemotherapy near EOL was shorter than for other patients [Citation4,Citation24]. One can assume that they did not gain a survival benefit from chemotherapy near EOL, and since they were more often admitted to and died in hospital, late chemotherapy might have a an overall negative impact. It is, however, possible that the reason for more hospitalizations was that these patients had more cancer symptoms, leading to more cancer therapy during EOL.
When GPS was assessable, it was significantly associated with chemo- or radiotherapy the last 30 days, suggesting that this index might be of value when considering palliative cancer therapy. An advantage of GPS is that it is easily assessed from objectively measurable blood values, whereas PS relies more on the individual physician's judgment.
Similar to our study, there are several reports showing that patients referred to a PCU receive less chemotherapy near EOL [Citation1,Citation10,Citation25]. In one study, patients with advanced NSCLC randomized to early contact with a PCU even had a longer survival than patients receiving standard care despite less chemotherapy [Citation4]. Thus, integrating palliative care into standard oncology care is recommended [Citation23]. One explanation could be that patients at a PCU achieve better symptom control [Citation4]. Another possibility is that personnel at a PCU more often discuss limitations of cancer therapy, which might make it easier to discontinue cancer therapy when the chance of a clinically relevant benefit becomes small [Citation18].
The main limitations to our study were that it was retrospective; data (such as PS and GPS) were not documented in all cases; we were not able to assess other factors that might influence the use of cancer therapy, such as comorbidity, travel time to the hospital and personal preferences; whether treatment was administered according to national guidelines; or whether the patients experienced symptom relief during EOL. Furthermore, we have no information about patients who were not referred to our institution. The main strength of our study is that we have collected data from individual medical records in a cohort selected on year of death only. All were treated at a single institution, which is the only cancer center in our county.
There are several possible explanations for why patients receive chemo- and radiotherapy during EOL. Common eligibility criteria for clinical trials are ‘Life expectancy of > 3 months’, PS 0–1 and absence of significant comorbidity. Many patients seen in the clinic are older, have poorer PS and more comorbidity, and it is possible that they do not benefit as much from the cancer treatment as patients participating in trials [Citation18,Citation19].
Another reason might be that physicians are not good at predicting survival [Citation14,Citation15]. PS is easy to assess and a robust prognostic factor [Citation16], but was only stated in 47% of patients when the last cancer therapy was initiated. Despite less use of chemotherapy during EOL than in other reports, too many patients, in our opinion, received such therapy. Our study suggests that the proportion can be reduced by following existing guidelines. PS should be assessed in all patients, and palliative chemotherapy for solid tumors should mainly be offered patients with a good PS of 0–1 [Citation2], unless they have a chemo-sensitive disease or as part of a clinical trial. Third-line regimens should only be administered when there is evidence of a clinical benefit. Physicians should pay more attention to estimating prognosis when considering chemotherapy or radiotherapy; assessing GPS might be a valuable tool. More studies evaluating the benefit of palliative cancer therapy – especially radiotherapy – in very advanced disease appears to be needed.
In conclusion, 18% of patients received chemo- or radiotherapy within 30 days of death at our cancer center. The proportions varied with age, cancer type and whether they were referred to the PCU, and were highest in patients with the shortest survival time. More attention to estimation of survival time when considering palliative therapy in patients with advanced cancer – possibly aided by assessing GPS– might be of help to improve practice.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andreis F, Rizzi A, Rota L, Meriggi F, Mazzocchi M, Zaniboni A. Chemotherapy use at the end of life. A retrospective single centre experience analysis. Tumori 2011;97:30–4.
- Goncalves JF, Goyanes C. Use of chemotherapy at the end of life in a Portuguese oncology center. Support Care Cancer 2008;16:321–7.
- Murillo JR, Jr., Koeller J. Chemotherapy given near the end of life by community oncologists for advanced non-small cell lung cancer. Oncologist 2006;11:1095–9.
- Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. New Engl J Med 2010;363:733–42.
- Tang ST, Liu TW, Shyu YI, Huang EW, Koong SL, Hsiao SC. Impact of age on end-of-life care for adult Taiwanese cancer decedents, 2001–2006. Palliat Med 2012;26:80–8.
- Yun YH, Kwak M, Park SM, Kim S, Choi JS, Lim HY, et al. Chemotherapy use and associated factors among cancer patients near the end of life. Oncology 2007;72:164–71.
- Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 2004;22:315–21.
- Earle CC, Landrum MB, Souza JM, Neville BA, Weeks JC, Ayanian JZ. Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 2008;26:3860–6.
- Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end-of-life cancer care in the universal health care system of Ontario, Canada. J Clin Oncol 2011;29:1587–91.
- Gonsalves WI, Tashi T, Krishnamurthy J, Davies T, Ortman S, Thota R, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran's Affairs cancer population. J Palliat Med 2011;14:1231–5.
- Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol 2013;31:80–7.
- Kapadia NS, Mamet R, Zornosa C, Niland JC, D’Amico TA, Hayman JA. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer 2012; 118:4339–45.
- Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in end-stage cancer patients: Reality or myth? Cancer 2010;116:3251–6.
- Christakis NA, Lamont EB. Extent and determinants of error in doctors’ prognoses in terminally ill patients: Prospective cohort study. Br Med J 2000;320:469–72.
- Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. Br Med J 2003; 327:195–8.
- Glare P, Sinclair C, Downing M, Stone P, Maltoni M, Vigano A. Predicting survival in patients with advanced disease. Eur J Cancer 2008;44:1146–56.
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40.
- Smith TJ, Hillner BE. Bending the cost curve in cancer care. New Engl J Med 2011;364:2060–5.
- Quill TE. Perspectives on care at the close of life. Initiating end-of-life discussions with seriously ill patients: Addressing the ‘elephant in the room’. JAMA 2000;284: 2502–7.
- Liu TW, Chang WC, Wang HM, Chen JS, Koong SL, Hsiao SC, et al. Use of chemotherapy at the end of life among Taiwanese cancer decedents, 2001–2006. Acta Oncol 2012;51:505–11.
- Asola R, Huhtala H, Holli K. Intensity of diagnostic and treatment activities during the end of life of patients with advanced breast cancer. Breast Cancer Res Treat 2006;100: 77–82.
- Colla CH, Morden NE, Skinner JS, Hoverman JR, Meara E. Impact of payment reform on chemotherapy at the end of life. Am J Managed Care 2012;18:e200–8.
- Steenland E, Leer JW, van Houwelingen H, Post WJ, van den Hout WB, Kievit J, et al. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 1999;52:101–9.
- Saito AM, Landrum MB, Neville BA, Ayanian JZ, Earle CC. The effect on survival of continuing chemotherapy to near death. BMC Palliat Care 2011;10:14.
- Magarotto R, Lunardi G, Coati F, Cassandrini P, Picece V, Ferrighi S, et al. Reduced use of chemotherapy at the end of life in an integrated-care model of oncology and palliative care. Tumori 2011;97:573–7.
