Abstract
Background. A large proportion of patients with pancreatic cancer presents with metastatic disease. We conducted a population-based study to evaluate trends in treatment and survival of patients with metastatic pancreatic cancer.
Methods. We included all patients diagnosed with pancreatic cancer between 1993 and 2010 in the South of the Netherlands (N = 3099). Multivariable logistic regression analysis was conducted to evaluate trends in treatment with chemotherapy. Crude overall survival according to period of diagnosis was analyzed, and independent risk factors for death were identified.
Results. Forty-eight percent of the patients (N = 1494) were diagnosed with metastatic disease. The percentage of patients being diagnosed with metastatic disease increased during the study period from 35% in 1993–1996 to 59% in 2009–2010 (p < 0.0001). Overall, 18% of these patients received chemotherapy. The prescription of palliative chemotherapy almost tripled from 10% to 27% (p < 0.0001). Treatment largely depended on age, ranging from 38% among patients aged < 50 years [compared to 60–69 years: adjusted odds ratio (ORadj) 2.5 (95% CI 1.4–4.2)] to 1% among patients aged ≥ 80 years [compared to 60–69 years: ORadj 0.04 (95% CI 0.0–0.2)]. Patients were more likely to receive chemotherapy if they had a high socioeconomic status [ORadj 2.0 (95% CI 1.3–3.1)], and if diagnosis was pathologically verified [no verification vs. verification: ORadj 0.3 (95% CI 0.2–0.5)]. The administration of chemotherapy varied widely between 10 hospitals (5–34%, p < 0.0001). The median overall survival of patients with metastatic pancreatic cancer remained 9–11 weeks.
Conclusion. A growing proportion of pancreatic cancer patients presented with metastatic disease. Usage of palliative chemotherapy increased over time, but median survival remained 9–11 weeks. In the near future, it should be evaluated if the recently introduced regimens have an impact on population-based survival.
Pancreatic cancer is a devastating disease, with a one-year survival rate ranging from 12% to 28% in Europe [Citation1]. It is characterized by aggressive tumor biology with early metastases. As a result, 95% of pancreatic cancer patients eventually die of the disease within five years.
The only curative treatment modality for pancreatic cancer is surgery. In the last decades, the survival for patients with resectable pancreatic cancer increased due to technical advances in surgical treatment as well as advances in other surgery-related factors, such as the establishment of high volume centers and implementation of adjuvant chemotherapy [Citation2,Citation3].
However, about 80% of the patients with pancreatic cancer is diagnosed with locally advanced or metastatic disease and does not qualify for surgical treatment. Furthermore, the majority of patients who are treated with pancreatic surgery eventually relapses and develops local recurrence or distant metastases [Citation2,Citation4].
Population-based data on treatment and outcome of metastatic pancreatic cancer are scarce. Recently, data from the US Surveillance, Epidemiology and End Result registry (SEER) showed a modest increase in median overall survival of patients with metastatic pancreatic cancer in the years 1988–2008 [Citation5].
We conducted a population-based study in order to evaluate whether the proportion of patients diagnosed with metastatic disease shifted over time, whether treatment patterns changed and to what degree this had an effect on median survival in the South of the Netherlands.
Methods
Data collection
For the present study we used data from the Eindhoven Cancer Registry (ECR), maintained by the Comprehensive Cancer Centre South. The ECR is a population-based registry in the Southern part of the Netherlands. The registry area comprises about 2.4 million inhabitants and encompasses 10 community hospitals, two radiotherapy institutions and six pathology departments. The area does not contain university or specialized cancer hospitals. Information on patient, tumor and treatment characteristics was routinely extracted from medical records by trained registrars operating on behalf of the ECR.
Our study included all patients who were diagnosed with a neoplasm of the pancreas [International classification of Disease for Oncology (ICD-O), 2nd edition, topography code 157 and 3rd edition, code C25] between 1 January 1993 and 31 December 2010. We decided to restrict the morphology to adenocarcinoma and excluded patients with neuroendocrine tumors, sarcomas or blastomas of the pancreas. However, tumors without histological confirmation were included.
The registrars classified all adenocarcinomas according to the Tumor Lymph Node Metastasis (TNM) classification and staged them following the recommendations of the International Union Against Cancer in the respective period. The clinical Extent of Disease (cEOD) was used for staging if the tumor was not histologically confirmed. We categorized tumors as locoregional (confined to the pancreas, with or without extension to surrounding organs or locoregional lymph nodes) or metastatic pancreatic cancer.
Vital status of patients was assessed at 1 January 2012 through linkage with civil municipal registries and the central bureau for genealogy. The latter is an institution that collects data on all deceased Dutch citizens. Survival was calculated based on all-cause mortality.
Statistical analysis
We described proportions of patients who received chemotherapy according to gender, age, socioeconomic status, number of comorbid conditions, histologic subtype, site of metastases, period of diagnosis and hospital of treatment (A–J). Differences in the administration of systemic chemotherapy between subgroups were tested by means of a χ2-test. Trends in treatment across the five periods (1993–1996, 1997–2000, 2001–2004, 2005–2008 and 2009–2010) were analyzed by means of a Cochran-Armitage trend test. Furthermore, the independent influence of these variables on the administration of chemotherapy was evaluated by means of a logistic regression analysis. Missing values were included as separate dummies in the analyses in order not to lose statistical power.
Survival time was defined as the time from diagnosis to death or 1 January 2012, for patients who were still alive. The significance of differences between survival curves were evaluated by means of a log rank test. Independent risk factors for death were discriminated by multivariable proportional hazard regression modeling. In order to investigate the effect of chemotherapy on the hazard ratios (HRs) of dying according to period of diagnosis and hospital of diagnosis, the model was run with and without treatment variable (chemotherapy yes vs. no).
SAS Statistical software (version 9.3, SAS institute, Cary, NC, USA) was used to perform the statistical analyses. For all analyses, a two-sided p-value < 0.05 was considered statistically significant.
Results
A total of 3099 patients were diagnosed with pancreatic cancer between 1 January 1993 and 31 December 2010. Forty-eight percent of the patients (N = 1494) were diagnosed with metastatic disease (pathological or clinical). This percentage increased over time, from 35% in 1993–1996 to 59% in 2009–2010 (p < 0.01).
General characteristics by period are depicted in . Fifty-five percent of our study population was male and 68% of the patients had their diagnosis confirmed by pathological examination. The median age at time of diagnosis was 68 years (range 32–99). Overtime the proportion of elderly patients diagnosed with metastatic pancreatic cancer increased.
Table I. Characteristics of patients with metastatic pancreatic cancer, by period of diagnosis (N = 1494).
The liver (76%) and the peritoneal cavity (18%) were the most common metastatic sites. Twenty-four percent of the patients had metastases in two or more organs, this proportion increased overtime. Patients with liver metastases were more likely to have multiple organs affected compared to patients with non-liver metastases (p = 0.03).
The use of chemotherapy among patients with metastatic pancreatic cancer increased, from 10% in 1993–1996 to 27% in 2009–2010 (p < 0.001) (). Several factors influenced the probability of receiving chemotherapy. shows the crude proportions of patients treated with chemotherapy according to relevant patient and tumor characteristics, and the adjusted odds of being treated with chemotherapy. Chemotherapy was administered more often to younger patients and patients with a high socioeconomic status (SES). In contrast, older patients and patients without pathological confirmation received chemotherapy less frequently. Furthermore, there was a large inter-hospital variation in the prescription of chemotherapy (5–34%); this variation was not related to the size of the hospital.
Figure 1. Administration of chemotherapy for patients diagnosed with metastatic pancreatic cancer between 1993 and 2010 in the southern Netherlands according to period of diagnosis (N = 1494).
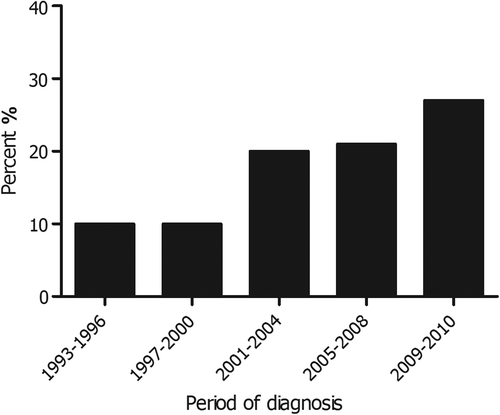
Table II. Crude percentages, unadjusted and adjusted odds for receiving chemotherapy among patients diagnosed with metastatic pancreatic cancer between 1993 and 2010 in the southern Netherlandsa (N = 1494).
The overall survival of patients with metastatic pancreatic cancer did not change in the course of time. The prognosis remained dismal, with one-year survival rates between 4% and 7% and a median overall survival between 9 and 11 weeks (). Patients treated with palliative chemotherapy exhibited a median overall survival of 25 weeks (one-year survival rate 17%), compared to eight weeks in those not treated with chemotherapy (one-year survival rate 3%) (). Other beneficial prognostic factors identified by multivariable survival analysis were female sex, single site metastases in extra regional lymph nodes, and dissemination limited to the lungs. Factors associated with poor survival included older age (70–79 years or 80+). Patients without microscopically verified pancreatic cancer carried the same dismal prognosis as patients with verified pancreatic cancer.
Figure 2. Overall survival of patients diagnosed with metastatic pancreatic cancer between 1993 and 2010 in the southern Netherlands according to period of diagnosis (N = 1494).
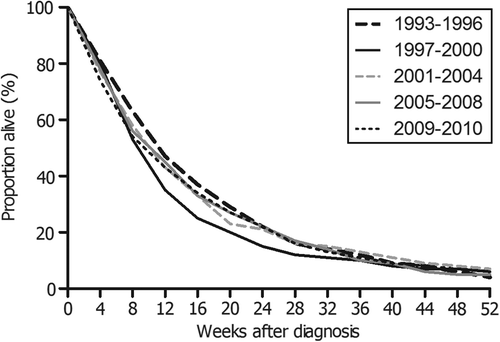
Table III. Crude median overall survival, crude 1-year survival, unadjusted and adjusted hazard ratios for patients diagnosed with metastatic pancreatic cancer between 1993 and 2010 in the southern Netherlandsa (N = 1494).
Without adjustment for chemotherapy in the multivariable survival analysis, the risk of dying remained similar across the different study periods. After adjustment for chemotherapy the risk of dying increased significantly over time. Patients diagnosed in hospital B and G had a better survival, but after adjustment for chemotherapy this difference was not observed anymore.
Discussion
This population-based study showed that an increasing proportion of pancreatic cancer patients presented with metastatic disease. The administration of palliative chemotherapy in these patients increased drastically, and large inter-hospital variations in the administration were noted. In the course of time, the population-based median overall survival remained dismal.
The increasing proportion of pancreatic cancer patients to present with metastatic disease is consistent with previous reports [Citation6,Citation7]. The improvements in the accuracy of the current computed tomography (CT) scans and the development of new diagnostic tools are major contributing factors. Once (metastatic) pancreatic cancer is suspected on imaging studies, pathologic confirmation is required to establish the definitive diagnosis. In 68% of the patients with metastatic pancreatic cancer the definitive diagnosis was verified by pathological examination. Low overall proportions of verification are common in pancreatic cancer. The EUROCARE-4 study, a study that gathered data from 93 European cancer registries between 1995 and 2002, found a verification rate of 63% (range 30–91%) in all patients with pancreatic cancer [Citation8]. Our data suggest that the rate is especially low in non-metastatic pancreatic cancer. Obtaining tissue in the absence of metastases can be difficult, since the pancreas is situated in the retroperitoneum and the abundant presence of desmoplastic stroma in pancreatic tumors may hamper the identification of malignant tumor cells [Citation8,Citation9]. In case of metastatic disease, healthcare practitioners often refrain from diagnostic biopsies in patients who are considered unfit for systemic treatment. Although in several population-based studies, pathologically unverified tumors were not taken into account [Citation10,Citation11], we included these patients into our study, in order to obtain a true reflection of the outcome of daily clinical practice in pancreatic cancer care. Since the overall survival of patients with histologically unverified pancreatic cancer was poor, the likelihood that these patients indeed suffered from pancreatic cancer is high. We believe it is of clinical relevance to report on the outcome of these patients, as they constitute a significant proportion of pancreatic cancer patients.
Although no considerable progress has been made in the chemotherapeutic treatment of metastatic pancreatic cancer during the study period, the prescription of chemotherapy increased from 10% 1993–1996 to 27% 2009–2010. Since 1997, gemcitabine monotherapy has been the reference regimen for patients with metastatic pancreatic cancer. Although initial studies suggested a low response rate (6–11%), gemcitabine was approved for first-line treatment on the basis of significant improvement in ‘clinical benefit’ (pain relief, improved performance status, or both) and prolongation of survival (5.6 months in gemcitabine-treated patients vs. 4.4 months in 5-FU-treated patients) [Citation12]. In numerous trials over the years, many different (drug) regimens have been tested. Until recently, none of these trials demonstrated a statistically significant survival benefit, except for gemcitabine plus erlotinib which was associated with a very modest, clinically irrelevant increase in overall survival of two weeks [Citation13]. In the course of time, the prescription of palliative chemotherapy increased drastically, possibly because physicians became more familiar with the use of chemotherapy in pancreatic cancer after the publication by Neoptolemos et al. [Citation14] in 2001 of adjuvant gemcitabine chemotherapy in patients with resected pancreatic cancer [Citation15]. A similar phenomenon has been observed in patients with metastasized gastric cancer, after the introduction of perioperative chemotherapy [Citation16].
In our study the odds of receiving chemotherapy was influenced by several patient- and tumor-related factors. First, an inverse relationship between the administration of chemotherapy and age was found, which is consistent with findings from previous reports [Citation17]. Second, patients with a higher socioeconomic status were more likely to receive chemotherapy [Citation6,Citation7]. This treatment selection according to SES has also been described by Krzyzanowska et al. [Citation17] for elderly patients with advanced pancreatic cancer and by Chueng et al. [Citation18] for a cohort of patients living in the state of Florida. Third, patients without microscopically verified pancreatic cancer had lower odds for receiving chemotherapy. This is not surprising, since most guidelines recommend ‘a positive biopsy’ before systemic treatment is started, and a biopsy can only be omitted when patients are not fit for treatment.
We observed a large hospital variation in prescription of palliative chemotherapy after adjusting for casemix. In the Netherlands, the treatment guidelines state that palliative chemotherapy should be considered in case of metastatic pancreatic cancer [Citation19]. However a recent study found that this consideration is strongly influenced by the physicians’ amount of experience and their judgment about the benefit of treatment and performance status of the patient [Citation20]. This could explain the large hospital variation observed in our study.
In the present study, the median overall survival for patients who were treated with chemotherapy was 25 weeks compared to eight weeks for untreated patients. In our multivariable proportional hazard analyses, chemotherapy was found to reduce the risk of death by 61%. This is high in comparison with the results from a meta-analysis of chemotherapy for locally advanced and metastatic pancreatic cancer. In this meta-analysis the risk of death was reduced by 36% [Citation21]. The difference can be explained by the observational character of our study and underscores the influence of selection: fitter patients or patients with less advanced disease, i.e. those with a better overall survival beforehand, more often received chemotherapy. Unfortunately information on additional confounders such as performance status and disease related symptoms are lacking due to the population-based nature of our data.
Although patients treated with systemic chemotherapy had a better overall survival, increased administration of chemotherapy did not improve population-based overall survival. Perhaps, population-based improvement was not achieved because the proportion of metastatic pancreatic cancer patients treated with chemotherapy was too small, or the impact of the regimen on overall survival was too little to achieve population-based improvement. However, in our multivariable survival analysis we found that before inclusion of chemotherapy (yes vs. no) in to the model survival significantly worsened in the course of time, an effect which disappeared after adding this variable. This might be interpreted as the increased rates of chemotherapy have had a beneficial effect on an otherwise even increasingly detrimental prognosis of this patient group.
Fortunately treatment options for metastatic pancreatic cancer increased, with the introduction of new drugs and multidrug regimens. For instance FOLFIRINOX, introduced in 2011, was the first regimen to result in a median overall survival of almost one year in patients with metastatic pancreatic cancer [Citation22,Citation23]. The regimen is, however, more toxic than gemcitabine and may therefore only be administered to younger patients with a good performance status [Citation22]. Recently, a new combination of nab-paclitaxel and gemcitabine was introduced by van Hoff et al. this regimen also showed a clinically meaningful improvement in overall survival of patients with metastatic pancreatic cancer and may be less toxic than FOLFIRINOX [Citation24]. As mentioned before, the impact of a regimen on population-based overall survival depends on the proportion of patients eligible to receive the regimen and the efficacy of the regimen. Although FOLFIRINOX is currently the most effective treatment in metastatic pancreatic cancer, its use is restricted to a selected group of patients [Citation22]. Nab-paclitaxel plus gemcitabine can be used more widely, but improved the median overall survival with only 1.8 months compared to gemcitabine alone [Citation24]. It is questionable if these regimens have enough impact to improve population-based overall survival in the near future.
However, considering these new treatment modalities the aforementioned large hospital variation in chemotherapy prescription rates might be of concern. The availability of new, more toxic, treatment regimens warrants more appropriate selection of patients, and possibly treatment by a medical oncologist with more experience with these regimens. It may be hypothesized that – similar to the centralization of surgical care for patients with resectable pancreatic cancer which has had tremendous impact on treatment outcome, and is now generally accepted in Europe [Citation2,Citation25] – also patients eligible for chemotherapeutical treatment might benefit from centralization by medical oncologists. Local tumor boards might offer a solution until centralization is achieved.
In conclusion, the median survival of patients with metastatic pancreatic cancer between 1993 and 2010 remained 10 weeks in spite of a significant increase in the proportion of patients being treated with palliative chemotherapy. In the near future, it should be evaluated if the recently introduced regimens have an impact on population-based survival.
Acknowledgments
The authors thank the registration team of the Eindhoven Cancer Registry for their dedicated data collection. Furthermore, we thank all the contributing hospitals: Amphia Hospital, Breda; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond and Deurne; Hospital Bernhoven, Uden; Jeroen Bosch Hospital, ‘s Hertogenbosch; Máxima Medical Centre, Eindhoven and Veldhoven; St. Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; TweeSteden Hospital, Tilburg and Waalwijk; VieCuri Hospital, Venlo and Venray; Instituut Verbeeten, Tilburg.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Moller H, Linklater KM, Robinson D. A visual summary of the EUROCARE-4 results: A UK perspective. Br J Cancer 2009;101(Suppl 2):S110–4.
- Lemmens VE, Bosscha K, van der Schelling G, Brenninkmeijer S, Coebergh JW, de Hingh IH. Improving outcome for patients with pancreatic cancer through centralization. Br J Surg 2011;98:1455–62.
- Seufferlein T, Bachet JB, Van Cutsem E, Rougier P. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23(Suppl 7):vii33–40.
- Pancreatic cancer in the UK. Lancet 2011;378:1050.
- Worni M, Guller U, White RR, Castleberry AW, Pietrobon R, Cerny T, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: A trend analysis using the surveillance, epidemiology, and end results registry from 1988 to2008. Pancreas 2013;42: 1157–63.
- Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol 2007;14:1320–6.
- Sharp L, Carsin AE, Cronin-Fenton DP, O’Driscoll D, Comber H. Is there under-treatment of pancreatic cancer? Evidence from a population-based study in Ireland. Eur J Cancer 2009;45:1450–9.
- De Angelis R, Francisci S, Baili P, Marchesi F, Roazzi P, Belot A, et al. The EUROCARE-4 database on cancer survival in Europe: Data standardisation, quality control and methods of statistical analysis. Eur J Cancer 2009;45: 909–30.
- Bjerregaard JK, Mortensen MB, Schonnemann KR, Pfeiffer P. Characteristics, therapy and outcome in an unselected and prospectively registered cohort of pancreatic cancer patients. Eur J Cancer 2013;49:98–105.
- Boyd CA, Benarroch-Gampel J, Sheffield KM, Cooksley CD, Riall TS. 415 patients with adenosquamous carcinoma of the pancreas: A population-based analysis of prognosis and survival. J Surg Res 2012;174:12–9.
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. Survival among patients with adenocarcinoma of the pancreas: A population-based study (United States). Cancer Causes Control 2006;17:403–9.
- Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol 1997;15:2403–13.
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: A phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25: 1960–6.
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001;358:1576–85.
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA 2007;297:267–77.
- Bernards N, Creemers GJ, Nieuwenhuijzen GA, Bosscha K, Pruijt JF, Lemmens VE. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann Oncol 2013;24:3056–60.
- Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: Population-based practices and effectiveness. J Clin Oncol 2003;21: 3409–14.
- Cheung MC, Yang R, Byrne MM, Solorzano CC, Nakeeb A, Koniaris LG. Are patients of low socioeconomic status receiving suboptimal management for pancreatic adenocarcinoma? Cancer 2010;116:723–33.
- National Working Group on Gastrointestinal Cancers. Guideline: Pancreatic cancer. The Netharlands: Comprehensive Cancer Centre; 2011.
- Schildmann J, Tan J, Salloch S, Vollmann J. ‘Well, I think there is great variation..’: a qualitative study of oncologists’ experiences and views regarding medical criteria and other factors relevant to treatment decisions in advanced cancer. Oncologist 2013;18:90–6.
- Sultana A, Smith CT, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607–15.
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med 2011;364: 1817–25.
- Ko AH. FOLFIRINOX: A small step or a great leap forward? J Clin Oncol 2011:29:3727–9.
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med 2013;369: 1691–703.
- de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, et al. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg 2011;99:404–10.
