To the Editor,
In most institutions, standard treatment of T2-T3 rectal adenocarcinoma is radical surgery using total mesorectal excision (TME) with or without neo-adjuvant treatment [radiotherapy or chemoradiotherapy (CRT)]. Abdomino-perineal excision (APE) is considered by most patients as a severe mutilation but even with low anterior resection (LAR) morbidity is not negligible [Citation1]. Further, postoperative mortality, especially in elderly patients is of great concern [Citation2]. To improve quality of life and individualize treatment, an increasing number of experienced colorectal surgeons are advocating organ preservation using CRT followed either by local excision (LE) [Citation3,Citation4] or only close surveillance after clinical complete response (cCR) usually in T2 or ‘early T3’ tumors [Citation5,Citation6]. In Sao Paulo, Habr-Gama has since many years been the pioneer of such an approach recommending careful evaluation of the clinical tumor response [Citation7] using a long interval after the end of CRT. In case of cCR, her strategy is to adopt a ‘watch and wait’ (W-W) policy [Citation8]. In Lyon, Papillon using contact x-ray brachytherapy (CXB) alone was able in the 1970s to achieve close to 90% cCR in more than 300 T1 N0 lesions and gave his name to this technique [Citation9]. Since the mid-1980s, CXB was combined with external beam radiation therapy (EBRT) in order to treat, mainly in inoperable patients, T2-3 tumors at higher risk of perirectal nodal extension [Citation10,Citation11]. Similar approaches were used in various French, British and American institutions [Citation12–15]. Since the mid-1990s, CXB was progressively abandoned for four reasons: the Philips RT 50 TM Contact machine was not manufactured anymore, technological innovations drove the interest of radiation oncologists towards three-dimensional (3D) image-guided radiotherapy, they progressively lost the clinical expertise of rigid rectoscopy and LE became the primary treatment of malignant polyps or T1N0 tumors. In 2009 a new Contact machine named Papillon 50TM, producing a similar 50 kV x-ray beam was introduced in UK and France and initiated a renaissance of this technique [Citation16]. We report here an overview of the use of CXB during a time period of 35 years by a homogeneous team of radiation oncologists working successively in CHU Lyon Sud and Centre Antoine Lacassagne in Nice. The report will focus exclusively on the combined treatment using CXB with EBRT to achieve organ preservation in T2 to early T3 rectal adenocarcinomas.
Material and methods
Cohort stratification
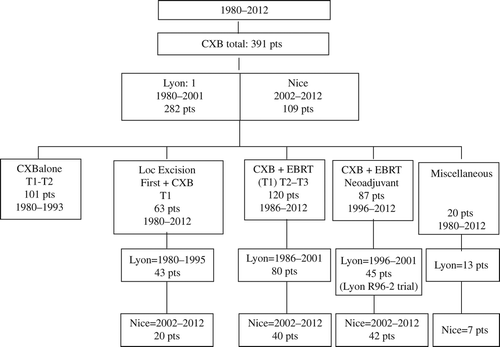
Between 1980 and 2012, a total of 391 patients have been treated in Lyon and Nice using CXB in five different situations ():
| 1) | CXB alone for 101 T1 (T2) N0 in the early period from 1980 to 1993 [Citation17]; | ||||
| 2) | adjuvant CXB after LE for 63 malignant polyps or T1 tumors during the whole period [Citation18,Citation19]; | ||||
| 3) | CXB combined with EBRT with curative and organ preservation intent in 120 patients with T2 and early T3 Nx M0 tumors [Citation11,Citation19,Citation20]. Few T1 with adverse histological features (poor differentiation, colloid component, venous invasion), all with high risk of lymph node invasion, have also been included. This report focuses on this cohort of patients treated between 1986 and 2012. Many of them were considered inoperable or at high surgical risk. | ||||
| 4) | CXB combined with EBRT (with or without concurrent chemotherapy) since 1996, in a neo-adjuvant setting before radical TME surgery for 87 operable patients presenting T3 (T2) Nx M0 distal tumors [Citation19,Citation21,Citation22]. A group of 45 of these patients were included in the Lyon R96-02 trial [Citation21,Citation22]. | ||||
| 5) | An additional small group of 20 miscellaneous patients treated using CXB often for palliation has never been published (). | ||||
Patients and tumors characteristics
Between 1986 and 2012, a total of 120 patients were treated with curative intent and for organ preservation using an association of CXB and EBRT. These patients were either medically inoperable (32 patients), at high surgical risk or had adamantly refused a permanent colostomy. Adenocarcinoma was proven by biopsy in all of them. Tumors were always located in the last 10 cm (from anal verge) of the rectum and accessible to digital rectal examination (DRE). Clinical staging was made with DRE and rigid rectoscopy in the knee chest position. An empty rectum was achieved using enema. A 2.5 cm endoscope in diameter either stainless steel or more recently disposable plastic (Legrand TM) was used to assess tumor appearance and measure its diameter. The 3 cm rectal applicator used at time of CXB treatment confirmed the measurements. Imaging was always required, initially until mid-1990s using only endorectal ultrasound (EUS) performed by dedicated gastroenterologists. More recently, magnetic resonance imaging (MRI) was performed routinely often combined with EUS and sometimes with positron emission tomography (PET). Search for metastasis was done initially with chest x-ray and liver ultrasound and later on with thoraco-abdomino-pelvic CT. Serum CEA was routinely measured. As this work-up was done by the same senior consultant (JPG) with the cooperation of a small group of radiation oncologists having a similar clinical approach of rectal cancer, tumor (T) classification was kept constant during this period of time. Characteristics of the patients are shown in .
Table I. Main characteristics of patients and tumors.
Treatments
Contact x-ray brachytherapy (CXB)
CXB was performed with the Philips RT 50TM until 2009. The x-ray beam energy was 50 Kvp. The source skin distance (SSD) was 4 cm. The rectal applicator diameter was 3 cm. In 10% of the patients, a local anesthesia using 20 cm3 of Lidocaine 2%TM was necessary to ease the introduction of the applicator. After downsizing the tumor, it was sometimes possible to use a 2 cm diameter applicator. The treatment was nearly always initiated with CXB delivering on average a dose of 85 Gy in 3 fractions over four weeks (D1: 30 Gy; D14: 30 Gy; D28: 25 Gy). Depending on the tumor shrinkage, a fourth session could be used for a total dose close to 100 Gy. Progressively, as the tolerance of this combined treatment was good, the dose of CXB was slightly increased to 90 Gy/3 fractions. Since 2009 in Nice, all CXB treatments were delivered using the Papillon 50TM machine which is quite similar to the Philips RT 50TM. The x-ray tube cooling, which is a key technological point for a high dose rate, is achieved with paraffin oil circuit and not with air as in the Philips machine, making it possible to have a smaller tube (21 mm diameter) easier to introduce through a smaller applicator, being more comfortable for the patient. There are three different sizes of applicator: 3, 2.5 and 2.2 cm in diameter. CXB was always initiated with the 3 cm applicator to encompass a larger volume and the 2.5 or 2.2 cm applicators were used only after significant (centripetally or concentric) shrinkage of the tumor. If the tumor was larger than 3 cm, CXB was initiated using two overlapping fields. The dose distribution, as displayed using the MC2 Plan Monte-Carlo software for treatment planning, shows that with a 3 cm applicator and a SSD of 3.8 cm, the 50% isodose is at 7 mm from the applicator end surface and is encompassing 5 cm3 volume [Citation23]. The dose rate depending on the applicator is between 15 and 20 Gy/minute. This CXB technique has been described in more detail [Citation11,Citation16].
External beam radiation therapy (EBRT)
EBRT was performed using linear accelerator with high energy photon of 18 mV energy and a three-field technique with wedges. Initially EBRT was performed with a 2D technique and since 1993 using a 3D conformal technique with multi-leaf collimators. The treated volume encompassed the gross tumor volume (GTV) and for clinical target volume (CTV), the entire mesorectum, presacral nodes and the latero-pelvic nodes (internal iliac). The obturator and external or common iliac nodes were never included in the CTV. The upper limit of CTV was at the level of the S1/S2 junction or for distal tumors S2/S3. Patients were treated in the prone position and the anal canal was outside the CTV using a radio-opaque marker at time of simulation. As the volume had no concave shape in the transverse axial plane, intensity-modulated radiation therapy (IMRT) was not deemed necessary. The planning target volume (PTV) extension was between 0.5 and 1 cm depending on the location of the tumor in the rectum. The volume of the 95% isodose of the ICRU point never exceeded 1 l. After 1993 the dose was prescribed and reported in the ICRU point (ICRU 60). The dose per fraction and total dose have changed over time. In the Lyon period total dose was 39 Gy in 13 fractions (3 Gy per fraction). A concomitant EBRT boost was administered with a ‘field within the field’ technique (8 × 8 cm or GTV + 1 cm extension) delivering four times 1 Gy, 6 hours before 3 Gy into the larger field. This accelerated schedule could be estimated biologically equivalent to 50–54 Gy in daily fractions of 2 Gy [Citation11]. In Nice where EBRT was progressively combined with concurrent chemotherapy the dose per fraction was 2 Gy and the total dose 50 Gy over five weeks with a cone down boost (shrinking field) after 44 Gy. During this time EBRT was started after CXB usually on Day 28 (or Day 21 in the early period) after the first CXB session.
Associated treatments
Three main treatments were associated with the combined CXB and EBRT.
Interstitial192 iridium implant
It was used for the majority of patients during the Lyon period. Four to six weeks after completion of EBRT, a boost was given using a low-dose rate 192 iridium interstitial implant. In case of distal tumor, a perineal template was used with an average of five wires of 5 cm length and 1 cm spacing. If the tumor was in middle rectum a ‘rectal fork’ technique with two parallel wires of 4 cm long was used. With both types of implants the dose prescribed according to the Paris system was 20 Gy over one day duration on average. During the Nice period, this iridium implant was restricted to a few distal T3 tumors invading the upper part of the anal canal more difficult to irradiate with CXB (especially if located in the posterior rectum).
Concurrent chemotherapy
It was used only for a few patients in Lyon after 1998 but it became routine treatment in Nice as soon as the results of the FFCD 9203 randomized trial showed that concurrent chemotherapy was, with an acceptable toxicity, able to increase local control of rectal adenocarcinoma. Since 2005, oral capecitabine 800 mg/m2 twice a day on radiotherapy days, has replaced intra-venous 5-fluorouracil (CAP 50 protocol).
Local excision (LE)
In the early 2000s, LE following nCRT became popular in the French surgical community for T2 tumors. It was then introduced in our strategy especially to treat such tumors in operable patients to avoid TME surgery and preserve the whole rectum. An overview of the different treatments is presented in .
Table II. Overview of treatments techniques and strategies.
Follow-up and clinical evaluation
During these 35 years, all patients were submitted to close surveillance by the same team of radiation oncologists. The tumor response was first monitored during the CXB treatment with an accurate measurement of the size of the tumor and description of its downsizing and evaluation with DRE of its mobility and consistence. The tumor response on Day 21 or 28 after two sessions of CXB is very important as a cCR at that time is highly predictive of good prognosis in terms of local control and survival [Citation9]. Following the Papillon recommendations, the definition of a cCR has always been the same: total disappearance of the tumor with no visible growth on rectoscopy and a supple mucosa (or non-suspicious slight induration) on DRE [Citation17]. Progressively, EUS and MRI have been added to evaluate tumor response and more recently PET. The first control after end of treatment was performed two months later and then every three or four months during the first two years and then every six months until the fifth year and then annually. Perirectal lymph nodes are evaluated with careful DRE and various imaging techniques mainly EUS and MRI. Local control was defined as the absence of any progressive malignant lesion in the pelvis. In case of local relapse an attempt at surgical salvage was made any time the patient was operable. Colonoscopy was required every year if possible and distant metastases were controlled with various imaging techniques (chest x-ray and liver ultrasound in the early period and more recently CT scan, MRI and/or PET). Toxicity was recorded using standard scoring systems (EORTC, SOMA LENT and recently CTCAE-NCI) from 0 to 5. Grade 3 corresponds to non-life threatening toxicity requiring hospitalization or an endoscopic treatment. Bowel function was evaluated with the MSKCC scoring system using categories where 3 is good and 4 excellent [Citation24].
Statistical analysis
The data presented here are a compilation of results already published in various separate articles. For the Lyon period we have pooled two published cohorts of patients, the first one with 63 patients between 1986 and 1998 [Citation11] and the second one with 17 patients between 1998 and 2001 [Citation20]. For the Nice period, we have pooled the data of the first cohort of 16 patients treated between 2002 and 2006 [Citation19] with 24 patients treated between 2007 and 2012. It has not been possible to put them in a single database and therefore the results should be considered more as a general estimation than an accurate measurement of clinical outcomes. Nevertheless they can provide a reasonably good overview of our clinical experience over 26 years of time in two institutions taking in consideration the inevitable evolution occurring in terms of patient's characteristics, cancer diagnosis and treatments.
Results
The main clinical results are presented in .
Table III. Overview of clinical outcomes in 120 patients separated in two cohorts treated in Lyon and then in Nice. *Kaplan-Meier estimation at 5 years.
Compliance with treatment was usually very good with no dose reduction or interruption either for CXB, EBRT or concurrent chemotherapy when given.
In the Lyon period the third session of CXB was usually given on Day 21 and the rate of cCR on that day was around 60%. During the Nice period the third session was delayed to Day 28. The rate of cCR was then 80% and close to 90% for T2 not exceeding 3 cm in diameter.
The rate of cCR was close to 95% in both cohorts two months after end of treatment. LE after CXB was performed in 13 patients in Nice with no severe surgical complication but with grade 3 pelvic pain in two patients lasting for three months.
During the Lyon period, the rate of local recurrence at five years was 27% and it was 14% in the Nice period (Kaplan-Meyer estimation). Most of these recurrences occurred during the first two years after completion of treatment and all were located at the site of the initial primary tumor in the rectal wall and the underlying fat. In 40% of cases a local recurrence was associated with distant metastases, either synchronously or rapidly developing after the local recurrence.
There was no detectable isolated perirectal lymph node recurrence. In four cases a local recurrence was associated with an image of perirectal nodal relapse. A few patients developed extra pelvic lymph node recurrences mainly along the inferior mesenteric vessels or para-aortic area.
In the Lyon period six operable patients with local recurrence underwent an APE and all were long-term survivors with ultimate pelvic control. In Nice two patients with a local relapse less than 3 cm in diameter were treated with LE or a second course of CXB and have been locally controlled with good bowel function.
In the Lyon period, 58 (72%) of 80 patients were locally controlled, either initially or after salvage treatment. For the operable patients this rate was 92%. In Nice an ultimate local control was seen in 39 of 40 patients.
All patients without radical surgery (either APE or LAR) and without a permanent diverting colostomy (which was performed in three patients) are considered as having preserved their rectum, sometimes with a rectal tumor still locally progressive but usually with an acceptable bowel function and quality of life not requiring a palliative diverting stoma. Of 120 patients, 111 (92.5%) were able to preserve their rectum.
Median follow-up time was 63 months in both groups. The five-year survival was 64% and 39% in the Lyon and Nice periods, respectively. The shorter survival in Nice is related to a significantly older population. Five-year cancer-specific survival was 72% and 70% in Lyon and Nice, respectively. In the elderly population (above 80 years of age) a relatively high number of patients died from inter-current disease but also from distant metastasis which occurred in Nice in 18% of cases at five years.
Toxicity and bowel function
The most frequent toxicity was rectal bleeding. It was caused by radiation-induced telangiectasia which develops on the rectal mucosa usually 6–18 months after the end of treatment in 50–70% of cases. After 2–4 years these occasional bleedings diminish and disappear. In 5% of the cases, plasma-argon coagulation was successful for bleeding control. Blood transfusion was exceptional. During the first year an ulceration could be seen on rectoscopy and felt on DRE especially when treating T3 tumors where it occurred in 33% of cases. Monthly controls showed the progressive healing of this ulceration over 3–10 months. Usually T2 tumors healed without rectal wall modification or with a rectal wall scar slightly firm on DRE. There was no occurrence of rectal stenosis or perforation.
In 75–85% of cases the ano-rectal function was judged by the patient as good or excellent. Rectal bleeding could generate anxiety but did not affect the quality of life. There was no incontinence for gas or stool and patient did not wear pad except ‘by precaution’. During the first year after treatment some patients described some urgency and frequent stools mainly in the morning but usually not affecting their daily activity and disappearing with time.
Prognostic factors
The most important prognostic factors affecting cCR, local control and survival was the size of the tumor. Tumors less than 3 cm in diameter had cCR at two months close to 95% and local control close to 90%. The rate of local recurrence increased progressively with diameter above 3 cm and especially more than 4 cm (T > 4 cm: 24 pts with 10 local recurrences). The five-year overall survival was in Lyon 75% for T > 3 cm and 39% for T > 4 cm. Circumferential extension of the tumor was also closely related to cCR and local control and both declined when the tumor is close to or exceed half the rectal circumference.
Discussion
This report has many and obvious limitations. It is a pooling of data with no individual case-specific statistics. Selection of patients has been on individual cases with some evolution with time as demonstrated by the differences between the two Lyon and Nice cohorts. Treatment was monocentric and has been modified over time. For these reasons, the results should be considered as a general estimation of clinical outcome of treatment of ‘early (T1) T2-T3’ rectal adenocarcinomas of the distal and middle rectum mostly in inoperable or high surgical risk patients. However, all patients have been managed with routine use of rigid rectoscopy and a homogenous tumor classification.
The main result of this historical overview is that CXB is feasible and can be combined with EBRT with or without concurrent chemotherapy and if necessary followed by LE for patients of any age with low toxicity. The rates of cCR at two months are good (95%). In the recent Nice period, the rate of local recurrence at five years was 14% and organ preservation on the long term could be achieved in close to 95% of patients. These improved outcomes when compared with the Lyon period can be explained by better selection of patients (fewer large tumor > 3 or 4 cm in diameter), moderate increase in the dose of CXB (90 Gy vs. 85 Gy) and EBRT (50 Gy vs. 45 Gy) and use of concurrent chemotherapy with capecitabine. In case of local failure salvage radical surgery is possible with no particular toxicity. One interesting point to notice is that the rate of isolated lymph node relapse is very low as in most clinical experiences with organ preservation [Citation3–5,Citation8,Citation13,Citation15] which probably shows that EBRT (dose 45–50 Gy) ± concurrent chemotherapy is efficient to control subclinical lymph node deposits present in 15–25% of these ‘early’ rectal tumors.
Similar results have been published from institutions in the US or France using the Philips RT 50 machine until 2000 [Citation14]. The Liverpool- Clatterbridge recent results with the Papillon 50 machine are similar and also very encouraging [Citation25].
The present results in 120 patients can be compared with the results observed in the 45 operable patients included in the Lyon R 96-02 randomized trial and treated between 1996 and 2001 in Lyon with CXB (85 Gy/3 factions/21 days) and EBRT (39 Gy/13 factions/19 days) followed by radical TME surgery [Citation21,Citation22]. The rate of cCR in these 45 patients was lower (29%) probably because the tumors were more advanced, the neo-adjuvant treatment less aggressive with no concurrent CRT and the interval to clinical evaluation shorter. The CXB boost could without toxicity significantly increase the rate of sphincter saving surgery. Few patients (10 pts) with cCR were able to preserve their rectum either after close surveillance (W-W: 7 pts) or LE (3 pts). It is not yet known if LE adds clinical benefit when compared with W-W strategy in case of cCR.
In conclusion, for patients presenting T2 and early T3 of the distal and middle rectum, CXB 50 kV combined with EBRT with or without concurrent chemotherapy achieves a high rate of clinical complete responses. Such a cCR evaluated mainly with DRE and endoscopy is a major end point if the aim is organ preservation. In inoperable patients this treatment appears as a good option. In operable patients the upcoming OPERA randomized trial should aim at confirming the Lyon R 96.02 results.
Declaration of interest: The authors report no conflicts of interest. Jean-Pierre Gérard is the Medical Advisor of Ariane Medical System.
References
- Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: Development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 2012;255:922–8.
- Rutten HJ, den Dulk M, Lemmens VE, van de Velde CJ, Marijnen CA. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9: 494–501.
- Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012;99:1211–8.
- Garcia-Aguilar J, Shi Q, Thomas CR, Jr., Chan E, Cataldo P, Marcet J, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: Preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384–91.
- Maas M, Beets-Tan RG, Lambregts DM, Lammering G, Nelemans PJ, Engelen SM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol 2011;29:4633–40.
- Heald RJ, Beets G, Carvalho C. Report from a consensus meeting: Response to chemoradiotherapy in rectal cancer – predictor of cure and a crucial new choice for the patient: On behalf of the Champalimaud 2014 Faculty for ‘Rectal cancer: When NOT to operate’. Colorectal Dis 2014;16:334–7.
- Habr-Gama A, Perez RO, Wynn G, Marks J, Kessler H, Gama-Rodrigues J. Complete clinical response after neoadjuvant chemoradiation therapy for distal rectal cancer: Characterization of clinical and endoscopic findings for standardization. Dis Colon Rectum 2010;53:1692–8.
- Habr-Gama A, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Sabbagh C, Lynn PB, et al. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: Impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys 2014;88:822–8.
- Papillon J. Intracavitary irradiation of early rectal cancer for cure. A series of 186 cases. Cancer 1975;36:696–701.
- Papillon J. Present status of radiation therapy in the conservative management of rectal cancer. Radiother Oncol 1990;17:275–83.
- Gerard JP, Chapet O, Ramaioli A, Romestaing P. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys 2002;54:142–9.
- Horiot JC, Roth SL, Calais G, Nabid A, Bone-Lepinoy MC, Loiseau D. The Dijon clinical staging system for early rectal carcinomas amenable to intracavitary treatment techniques. Radiother Oncol 1990;18:329–37.
- Aumock A, Birnbaum EH, Fleshman JW, Fry RD, Gambacorta MA, Kodner IJ, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: Results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys 2001;51:363–70.
- Gerard JP, Romestaing P, Chapet O. Radiotherapy alone in the curative treatment of rectal carcinoma. Lancet Oncol 2003;4:158–66.
- Sun Myint A, Grieve RJ, McDonald AC, Levine EL, Ramani S, Perkins K, et al. Combined modality treatment of early rectal cancer: The UK experience. Clin Oncol (R Coll Radiol) 2007;19:674–81.
- Gerard JP, Myint AS, Croce O, Lindegaard J, Jensen A, Myerson R, et al. Renaissance of contact x-ray therapy for treating rectal cancer. Expert Rev Med Devices 2011;8: 483–92.
- Gerard JP, Ayzac L, Coquard R, Romestaing P, Ardiet JM, Rocher FP, et al. Endocavitary irradiation for early rectal carcinomas T1 (T2). A series of 101 patients treated with the Papillon's technique. Int J Radiat Oncol Biol Phys 1996; 34:775–83.
- Gerard JP, Chapet O, Romestaing P, Favrel V, Barbet N, Mornex F. Local excision and adjuvant radiotherapy for rectal adenocarcinoma T1-2 N0. Gastroenterol Clin Biol 2000; 24:430–5.
- Gerard JP, Ortholan C, Benezery K, Ginot A, Hannoun-Levi JM, Chamorey E, et al. Contact X-ray therapy for rectal cancer: Experience in Centre Antoine-Lacassagne, Nice, 2002-2006. Int J Radiat Oncol Biol Phys 2008;72: 665–70.
- Gerard JP, Baulieux J, Doyen J, Gal J, Letouze E, Olschwang S, et al. Towards a ‘Lyon molecular signature’ to individualize the treatment of rectal cancer. Prognostic analysis of a prospective cohort of 94 rectal cancers T1-2-3 Nx MO to be the basis of a molecular signature. Cancer Radiother 2012; 16:688–96.
- Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, et al. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: The Lyon R96-02 randomized trial. J Clin Oncol 2004; 22:2404–9.
- Ortholan C, Romestaing P, Chapet O, Gerard JP. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int J Radiat Oncol Biol Phys 2012;83:e165–71.
- Croce O, Hachem S, Franchisseur E, Marcié S, Gérard J-P, Bordy J-M. Contact radiotherapy using a 50 kv x-ray system: Evaluation of relative dose distribution with the Monte Carlo code PENELOPE and comparison with measurements. Radiat Phys Chem 2012;81:609–17.
- Minsky BD, Cohen AM, Enker WE, Sigurdson E. Phase I/II trial of pre-operative radiation therapy and coloanal anastomosis in distal invasive resectable rectal cancer. Int J Radiat Oncol Biol Phys 1992;23:387–92.
- Sun Myint A. Contact radiotherapy for elderly patients with early low rectal cancers. Br J Hosp Med (Lond) 2013; 74:391–6.