Abstract
Background. Stereotactic body radiotherapy (SBRT) has emerged as an effective treatment for localized prostate cancer. However, prostate specific antigen (PSA) kinetics after prostate SBRT have not been well characterized. The purpose of this study was to analyze the trend in PSA decline following robotic SBRT from a prospective cohort of patients.
Material and methods. In total 175 patients were treated definitively for localized prostate cancer to a dose of 35–36.25 Gy in 5 fractions using robotic SBRT in the absence of androgen deprivation therapy (ADT). PSA and testosterone were collected at regular intervals following treatment and patients were assessed for biochemical failure and benign PSA bounce. A PSA nadir threshold of 0.5 ng/ml was used as a predictor of long-term disease-free survival. Multivariate logistic regression was used to assess the effect of disease specific covariates on the likelihood of achieving a PSA nadir less than threshold. PSA kinetics were analyzed a multi-component exponential model accounting for benign and malignant sources of PSA.
Results and conclusion. At a median follow-up of 3 years, 70% of patients achieved a PSA nadir below 0.5 ng/ml with a median PSA nadir of 0.3 ng/ml at a median time to nadir of 30 months. In our cohort, 36.2% experienced a benign PSA bounce. Absence of PSA bounce, initial PSA, and testosterone at the time of nadir proved to be significant predictors of achieving a PSA nadir below threshold. PSA kinetics after prostate SBRT were well described with a functional volume model with fitted half-lives of 4.4 and 14.8 months for malignant and benign sources of PSA, respectively. Patients treated with prostate SBRT experience an initial period of rapid PSA decline followed by a slow decline which will likely result in lower PSA nadirs after longer follow-up. The long-term disease specific impacts of these results remain to be determined.
Ultra-hypofractionated stereotactic body radiotherapy (SBRT) has become an effective treatment option for localized prostate cancer [Citation1]. Exploitation of the linear quadratic model and the relatively low α/β ratio that has been estimated for prostate cancer (∼1.5) has allowed for the delivery of large biologically effective doses to the prostate over a short 5-fraction treatment course [Citation2–4]. As multiple prospective clinical trials using this approach have matured, 2–5-year biochemical progression-free survival rates have shown similar efficacy to contemporary dose-escalated intensity modulated radiation therapy (IMRT) series with acceptable rates of acute and late toxicity [Citation5–9]. However, long-term comparability in terms of prostate cancer specific mortality remains to be determined.
Prostate specific antigen (PSA) has been heavily scrutinized regarding its utility as a screening test for the diagnosis of prostate cancer [Citation10–12]. However, PSA has proven to be an invaluable tool in monitoring disease progression and relapse in patients who have been diagnosed and treated for prostate cancer. Furthermore, dynamic changes in PSA following treatment including PSA nadir and time to nadir have been shown to be predictive in treatment response to conventionally fractionated radiation therapy treatment regimens. PSA data from patients treated with conventional external beam radiation therapy suggest that patients achieving a PSA below 4 ng/ml at one year following treatment have improved rates of long-term disease control and overall survival [Citation13], and recent studies have demonstrated superior clinical disease-free survival in patients achieving a PSA nadir below 0.5 ng/ml [Citation14,Citation15].
Recently, Anwar et al. [Citation16] compared post- treatment PSA decline in a small series of patients who received CyberKnife (CK) prostate SBRT for localized prostate cancer to a similarly matched cohort of patients treated with conventionally fractionated dose-escalated IMRT. Their results demonstrated significantly lower PSA nadirs in the SBRT group compared to the IMRT group with substantially different rates of PSA decline, suggesting fundamental radiobiological differences between the two treatment regimens. There was also an increase in frequency of benign PSA bounces noted in the group of patients who received SBRT compared to conventionally fractionated IMRT, however, given the small number of patients included in the study, statistical significance could not be determined. Furthermore, due to the limited availability of PSA data, a rigorous analysis of PSA kinetics could not be performed.
Several models of PSA kinetics after definitive radiotherapy have been proposed in the literature [Citation17–26]. Most of these models have utilized an exponential decay term to account for the time-dependent decline in PSA production with or without a corrective term for PSA production from benign cells. These models have been mostly been employed in conventionally fractionated definitive prostate radiotherapy cohorts in an attempt to correlate rate of PSA change to long-term clinical outcomes. Unfortunately, very little data exists regarding the kinetics of PSA decline following ultra-hypofractionated SBRT for localized prostate cancer, and no models exist that take into account the potential increased biological effectiveness of large dose per fraction radiotherapy.
Given the increased radiobiological effective dose predicted for ultra-hypofractionated SBRT treatment of the prostate, we used a functional volume model modified from Swanson et al. [Citation23] to account for the loss of contribution to PSA production from both malignant and benign prostate tissue. We then applied this model to a large prospective cohort of patients treated with CK SBRT in the absence of androgen deprivation therapy (ADT) in an effort to better understand the radiobiological effect of ultra-hypofractionated SBRT on PSA kinetics in localized prostate cancer.
Material and methods
We retrospectively reviewed the charts of patients treated definitively for localized prostate cancer using the CyberKnife Radiosurgical System (CK, Accuray) at Georgetown University Hospital from 2007 to 2012. In total 175 consecutive patients with at least 12 months of follow-up who received a dose of 35–36.25 Gy in 5 fractions prescribed to a PTV that included the prostate and proximal seminal vesicles were included. The majority of patients treated were in the low to intermediate D’Amico clinical risk groups with clinical T1c, Gleason grade 6–7, and median PSA of 5.7 ng/ml (). None of the patients included in this study received ADT.
Table I. Patient characteristics (n = 175).
Serum PSA and testosterone levels were evaluated prior to the start of treatment and then every three months for the first year followed by every six months for the next four years. The PSA detection limit was 0.1 ng/ml and 0.01 ng/ml using standard and ultrasensitive PSA tests, respectively. For the purpose of data analysis, all PSA values less than 0.1 ng/ml were assigned a value of 0.1 ng/ml. Biochemical failure was defined according to the ASTRO Phoenix definition of a 2 ng/ml or greater rise in PSA above nadir. Benign PSA bounce was defined as a 0.2 ng/ml or greater rise in PSA that then returned to previous nadir. A threshold value of 0.5 ng/ml was chosen as a conservative PSA nadir cutoff based on previous studies demonstrating its long-term prognostic value after definitive radiotherapy [Citation14,Citation15]. Logistic regression was used to examine correlations between initial PSA, prostate volume, presence of PSA bounce, time to nadir, initial testosterone, testosterone at the time of nadir, Gleason score, and clinical stage on the likelihood of achieving a PSA nadir less than 0.5 ng/ml. A similar approach was used to examine predictors for benign PSA bounce. All statistical group comparisons were made using the Wilcoxon signed rank test.
Modeling of PSA kinetics
In an effort to better understand the biological effects of ultra-hypofractionated SBRT on normal and malignant PSA producing prostate cells, a multi-component functional volume model accounting for both malignant and benign prostate cell function loss was derived as a modification of the models proposed by Vollmer et al. [Citation22] and Swanson et al. [Citation23]. In this model, we use PSA production as a surrogate for prostate cell functional capacity.
where Vxo is the functional pretreatment prostate volume, βx is the proportionality constant relating functional prostate volume to PSA production, and ρx and γ are the first order rate constants for functional volume decay and serum PSA clearance, respectively. The subscripts m and b are used to denote malignant and benign prostate cells, respectively. Due to limitations in the ability to quantify benign and malignant prostate tissue volumes radiographically, Vxo and βx terms were combined into single initial PSA production rate terms α and ε, where
and solving EquationEquation 1 for PSA(t), we obtain:
EquationEquation 2 represents our base model of PSA decline after ultra-hypofractionated SBRT. In the setting of radical prostatectomy where no prostate tissue remains, α and ε are set equal to zero, and the equation reduces to a single exponential decay EquationEquation 3
) which is consistent with previous radical prostatectomy series.
We can also simplify EquationEquation 2 by ignoring the separate contributions of benign and malignant prostate epithelium to PSA production, and combine them into a single term μ = βV to yield:
Individual patient PSA data was fit to these models using non-linear least squares regression to obtain the first-order rate constants. A boot strap analysis using 1000 equally sized random samples was used to obtain overall cohort model parameters and confidence intervals.
Results
At a median follow-up of three years, 12 patients met the ASTRO Phoenix definition of biochemical failure. Of these 12 patients, six patients have subsequent PSA values that have returned to or below previous nadir without intervention. For the remaining 163 patients treated with ultra-hypofractionated SBRT at our institution, serial PSA measurements following treatment demonstrate a rapid PSA decline over the first 12 months followed by a slower gradual decline to nadir over the next two years (), while mean serum testosterone showed little decline from baseline ().
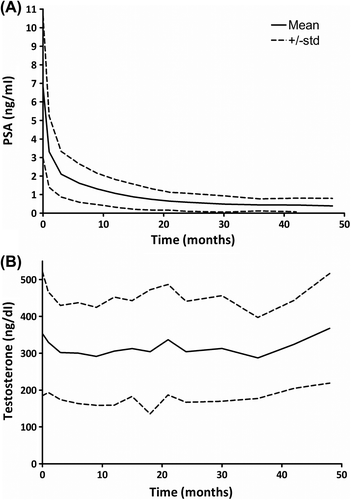
The median PSA nadir achieved was 0.3 ng/ml with a median time to nadir of 30 months ( and ). Benign PSA bounces were common with 36.2% of patients experiencing a post-treatment benign PSA bounce at a median time to bounce from the start of treatment of 15 months (). Patients experiencing a benign PSA bounce exhibited a statistically significant increase in median PSA nadir compared to patients without PSA bounce, 0.4 ng/ml vs. 0.2 ng/ml (p < 0.001) (). However, these patients continue to demonstrate declining PSA values with the majority of patients at their PSA nadir at the time of last follow-up.
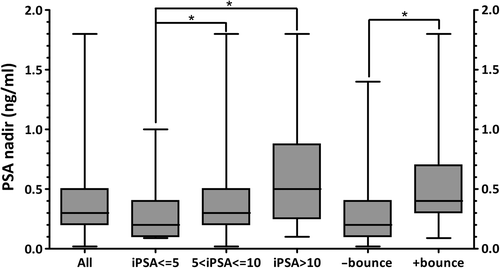
Table II. PSA Kinetics.
A PSA nadir threshold of 0.5 ng/ml was used to estimate the long-term likelihood of clinical disease-free survival. In our series 70.6% of patients have achieved a PSA nadir at or below this threshold (). The median PSA nadir of patients achieving a PSA below threshold was 0.2 ng/ml compared to 0.7 ng/ml for those with PSA nadir at or above threshold, p < 0.001. Selected clinical disease features including age, pretreatment PSA, clinical T stage, Gleason score, prostate volume, pretreatment testosterone, testosterone at the time of PSA nadir, time to PSA nadir, and presence of benign PSA bounce were examined as predictors for achieving a PSA nadir below 0.5 ng/ml using univariate logistic regression ( and ). Only absence of benign PSA bounce, pretreatment PSA, and testosterone at the time of nadir proved to be significant predictors of PSA nadir at a median follow-up of three years. These factors were tested together in a multivariate logistic model and remained significant with odds ratios of 0.29 (95% CI 0.14–0.61), 0.88 (95% CI 0.79–0.98) and 0.97 (95% CI 0.94, 0.99), respectively (). A similar approach was used to search for predictors of benign PSA bounce, however, no significant clinical factors were found.
Table III. Predictors of achieving PSA nadir < 0.5 ng/ml.
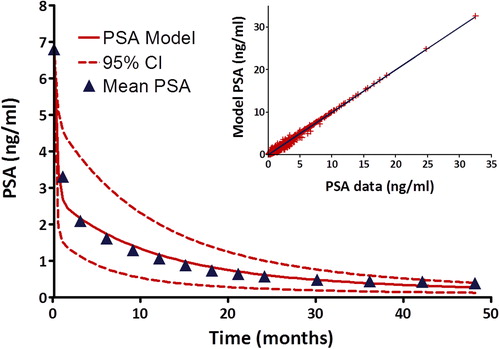
In an effort to better understand the radiobiological effect of ultra-hypofractionated SBRT on PSA kinetics in localized prostate cancer, we derived a functional prostate volume model to account for both benign and malignant sources of serum PSA (EquationEquation 2). This three-component exponential model was applied to our pooled cohort PSA data using non-linear least squares regression to obtain the pretreatment PSA production rates α and β, and the first order rate constants for the post-treatment time-dependent loss of benign and malignant PSA producing prostate functional volume, as well as serum PSA clearance. Comparison of the fitted model with our mean cohort post-treatment PSA data demonstrates excellent agreement with a correlation coefficient of 0.995 (). Assuming a shorter cell cycle time for malignant prostate cancer cells compared to benign prostate epithelium, our model predicts a loss of malignant prostate cancer cellular function with a half-life of 4.4 months (95% CI 3.3–6.5 months) compared to a benign prostate glandular functional half-life of 14.8 months (95% CI 11.8–19.7 months). Serum PSA half-life was determined to be approximately 4.8 days (95% CI 3.9–6.5 days). The ratio
, which represents the ratio of pretreatment malignant and benign sources of PSA production is approximately two-fold.
In order to test for over-fitting of our model, we repeated this fitting procedure for two other models with decreasing number of fitted parameters and then compared the AIC values. The first of these models grouped all sources of PSA production into one functional volume parameter and also accounted for serum PSA clearance (EquationEquation 4), while the second model only included a term for serum PSA clearance (EquationEquation 3
). Comparison of AIC values to our original three-component model (EquationEquation 2
) showed significant detriment in fit to the pooled cohort post-treatment PSA using either of these reduced parameter models.
Discussion
Early PSA responses in patients treated with ultra-hypofractionated SBRT for early localized prostate cancer have been encouraging [Citation5–9]. As studies mature and long-term data becomes available, a better understanding of the clinical disease features that may predict for durable treatment response will develop. In this study, we have attempted to rigorously analyze the early PSA responses obtained in our series at Georgetown University Hospital, and apply PSA nadir cutoffs (< 0.5 ng/ml) that have been shown to predict long-term disease-free survival in patients treated with conventionally fractionated definitive radiotherapy [Citation14,Citation15]. Using this approach, we demonstrate that patients treated with ultra-hypofractionated SBRT achieve median PSA nadirs that are lower than those typically reported in the conventionally fractionated literature [Citation27]. These results are consistent with a recent study reporting the ultra-hypofractionated prostate SBRT experience at UCSF, and suggest that durable clinical disease-free survival should be expected in the majority of our patients [Citation16].
Interestingly, of all the clinical disease features examined as potential predictors for achieving a PSA nadir below threshold, only initial PSA proved to be significant on multivariate analysis. Clinical T stage and Gleason score showed no association with PSA nadir, however, more advanced stage and high grade disease was clearly underrepresented in our cohort. Overall, testosterone levels remained mostly unchanged after treatment, but patients with lower testosterone values at the time of PSA nadir tended to achieve lower PSA nadirs as expected.
In our cohort, a significant number of patients experienced a benign PSA bounce (36.2%), which is similar to the incidence recently reported in the Winthrop University Hospital prostate SBRT experience (28%) [Citation28]. In that series, younger patient age was found to correlate with the development of benign PSA bounce after ultra-hypofractionated prostate SBRT. In contrast to those results, our multivariate logistic regression analysis showed no statistically significant association with any of the clinical factors examined, similar to previously reported conventionally fractionated studies [Citation29,Citation30]. We did find that patients experiencing a benign PSA bounce were more likely to have a higher PSA nadir, however, this is expected given the relatively short median follow-up of three years and the observation that the majority of patients continue to show declining PSA levels at the time of last follow-up. This suggests that transient increases in PSA after ultra-hypofractionated prostate SBRT should be interpreted cautiously, and that with longer follow-up, these patients will likely achieve PSA nadirs closer to those who did not experience a benign PSA bounce.
Many models for PSA decline after definitive treatment for prostate cancer have been proposed in the literature [Citation17–26]. In radical prostatectomy series, a single exponential decay model is most often used since all prostate tissue has been presumably removed and only serum clearance of PSA need be accounted for [Citation22]. For definitive radiotherapy, however, both malignant and benign prostate tissues remain, and the time dependent decline in serum PSA following treatment is more complex. Most models that have been described in the setting of definitive prostate radiotherapy have used either single or double exponential models, where the decline in prostate PSA production from benign and normal tissues have been grouped together in single parameter along with serum PSA clearance, or even added another parameter to account for regrowth of the benign prostate epithelium and resulting long-term increase in PSA production [Citation17,Citation20]. Given the ablative doses of radiation used in ultra-hypofractionated SBRT of the prostate, we postulated that in the post-treatment setting there will be a time dependent decrease in both the function of malignant and benign prostate cells, and that this decline should not be expected to occur over the same time scale due to the inherent biological differences between normal and malignant prostate epithelium.
We therefore derived our functional volume model based on models proposed by Vollmer and Swanson [Citation22,Citation23], and applied it to our ultra-hypofractionated prostate SBRT cohort. Our model shows excellent agreement to the post-treatment PSA data, and predicts for relatively long times to PSA nadir in these patients, given the observed half-lives of benign and malignant PSA production. The half-life of serum PSA clearance obtained from our model in slightly larger than that obtained in previous radical prostatectomy series (4.8 days vs. 3.2 days) [Citation31], however, is within reason. Due to current limitations in the ability to quantify malignant and benign prostate volume radiographically, it was necessary to combine initial prostate functional volume terms and their according PSA production proportionality constants into single fitted parameters. As prostate MRI imaging improves it will likely become possible to reliably quantify malignant and benign prostate volumes in future patients, thus potentially providing a better understanding of the relationship between tissue volume and cellular function using our model.
Similar to our results, Anwar et al. [Citation16], recently observed a similar trend in their ultra-hypofractionated SBRT cohort and was able to compare their results to a similarly matched group of patients that were treated with conventionally fractionated prostate radiotherapy. They showed a marked difference in PSA kinetics following treatment between the two modalities, with a significant change in PSA slope each year after treatment that persisted for the patients receiving SBRT. Unfortunately, their cohort contained a small number of patients with fewer PSA time points, so obtaining a statistically meaningful fit of our model for comparison would prove to be difficult. An important implication of the long time to PSA nadir demonstrated in our cohort and the Anwar study, is that any pathologic assessment of the prostate following definitive ultra-hypofractionated SBRT should be deferred for several years to avoid the possibility of a false positive biopsy and initiation of salvage treatments that may prove to be unnecessary to the patient and pose a significant risk of reduction in quality of life.
Conclusions
The majority of patients treated with robotic SBRT for localized prostate cancer achieve a PSA nadir less than 0.5 ng/ml. Benign PSA bounces are common and a significant proportion of biochemical failures will likely return to previous nadir or below with continued follow-up. Modeling of PSA kinetics suggests that patients treated with ultra-hypofractionated SBRT for localized prostate cancer experience an initial period of rapid PSA decline followed by a slow decline which continues at a median follow-up of three years. This will likely result in lower PSA nadirs after longer follow-up. The long-term prostate cancer specific and metastasis-free survival impacts of these results remain to be determined.
Declaration of interest: Sean Collins is a clinical consultant for Acuuray Inc.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Society for Radiation Oncology (ASTRO) Model Policy on Steretoactic Body Radiotherapy 2013. Available fromml: https://www.astro.org/uploadedFiles/Main_Site/Practice_Management/Reimbursement/2013HPcoding%20guidelines_SBRT_Final.pdf. Cited on 17th April, 2013.
- Dasu A, Toma-Dasu I. Prostate alpha/beta revisited – an analysis of clinical results from 14 168 patients. Acta Oncol 2012;51:963–74.
- Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: Alpha/beta = 1.4 (0.9–2.2) Gy. Int J Radiat Oncol Biol Phys 2011;82:e17–24.
- Proust-Lima C, Taylor JM, Secher S, Sandler H, Kestin L, Pickles T, et al. Confirmation of a low alpha/beta ratio for prostate cancer treated by external beam radiation therapy alone using a post-treatment repeated-measures model for PSA dynamics. Int J Radiat Oncol Biol Phys 2010;79:195–201.
- Chen LN, Suy S, Uhm S, Oermann EK, Ju AW, Chen V, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: The Georgetown University experience. Radiat Oncol 2013;8:58.
- Freeman DE, King CR. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol 2011;6:3.
- Katz AJ, Santoro M, Diblasio F, Ashley R. Stereotactic body radiotherapy for localized prostate cancer: Disease control and quality of life at 6 years. Radiat Oncol 2013;8:118.
- King CR, Brooks JD, Gill H, Presti JC, Jr. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2011;82:877–82.
- McBride SM, Wong DS, Dombrowski JJ, Harkins B, Tapella P, Hanscom HN, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma: Preliminary results of a multi-institutional phase 1 feasibility trial. Cancer 2011;118:3681–90.
- Slomski A. USPSTF finds little evidence to support advising PSA screening in any man. JAMA 2011;306:2549–51.
- Scherger JE. PSA screening: The USPSTF got it wrong. J Fam Pract 2013;62:616, 8.
- Lefevre M. PSA screening: The USPSTF got it right. J Fam Pract 2013;62:617, 9.
- Cheung R, Tucker SL, Kuban DA. First-year PSA kinetics and minima after prostate cancer radiotherapy are predictive of overall survival. Int J Radiat Oncol Biol Phys 2006;66: 20–4.
- Ray ME, Thames HD, Levy LB, Horwitz EM, Kupelian PA, Martinez AA, et al. PSA nadir predicts biochemical and distant failures after external beam radiotherapy for prostate cancer: A multi-institutional analysis. Int J Radiat Oncol Biol Phys 2006;64:1140–50.
- Zietman AL, Tibbs MK, Dallow KC, Smith CT, Althausen AF, Zlotecki RA, et al. Use of PSA nadir to predict subsequent biochemical outcome following external beam radiation therapy for T1-2 adenocarcinoma of the prostate. Radiother Oncol 1996;40:159–62.
- Anwar M, Weinberg V, Chang AJ, Hsu IC, Roach M, 3rd, Gottschalk A. Hypofractionated SBRT versus conventionally fractionated EBRT for prostate cancer: Comparison of PSA slope and nadir. Radiat Oncol 2014;9:42.
- Hanlon AL, Moore DF, Hanks GE. Modeling postradiation prostate specific antigen level kinetics: Predictors of rising postnadir slope suggest cure in men who remain biochemically free of prostate carcinoma. Cancer 1998;83:130–4.
- Semjonow A, Schmid HP. The rise and fall of PSA: Clinical implications of prostate specific antigen kinetics. Urol Res 2002;30:85–8.
- Denham JW, Lamb DS, Joseph D, Matthews J, Atkinson C, Spry NA, et al. PSA response signatures – a powerful new prognostic indicator after radiation for prostate cancer? Radiother Oncol 2009;90:382–8.
- Vollmer RT, Montana GS. The dynamics of prostate-specific antigen after definitive radiation therapy for prostate cancer. Clin Cancer Res 1999;5:4119–25.
- Vollmer RT. Dissecting the dynamics of serum prostate-specific antigen. Am J Clin Pathol 2010;133:187–93.
- Vollmer RT, Humphrey PA. Tumor volume in prostate cancer and serum prostate-specific antigen. Analysis from a kinetic viewpoint. Am J Clin Pathol 2003;119:80–9.
- Swanson KR, True LD, Lin DW, Buhler KR, Vessella R, Murray JD. A quantitative model for the dynamics of serum prostate-specific antigen as a marker for cancerous growth: An explanation for a medical anomaly. Am J Clin Pathol 2001;158:2195–9.
- Ritter MA, Messing EM, Shanahan TG, Potts S, Chappell RJ, Kinsella TJ. Prostate-specific antigen as a predictor of radiotherapy response and patterns of failure in localized prostate cancer. J Clin Oncol 1992;10:1208–17.
- Zagars GK, Pollack A. The fall and rise of prostate-specific antigen. Kinetics of serum prostate-specific antigen levels after radiation therapy for prostate cancer. Cancer 1993; 72:832–42.
- Kaplan ID, Cox RS, Bagshaw MA. A model of prostatic carcinoma tumor kinetics based on prostate specific antigen levels after radiation therapy. Cancer 1991;68:400–5.
- Cavanaugh SX, Kupelian PA, Fuller CD, Reddy C, Bradshaw P, Pollock BH, et al. Early prostate-specific antigen (PSA) kinetics following prostate carcinoma radiotherapy: Prognostic value of a time-and-PSA threshold model. Cancer 2004;101:96–105.
- Vu CC, Haas JA, Katz AE, Witten MR. Prostate-specific antigen bounce following stereotactic body radiation therapy for prostate cancer. Front Oncol 2014;4:8.
- Rosser CJ, Kamat AM, Wang X, Do KA, Sanchez-Ortiz RF, Kuban DA, et al. Is patient age a factor in the occurrence of prostate-specific antigen bounce phenomenon after external beam radiotherapy for prostate cancer? Urology 2005;66: 327–31.
- Rosser CJ, Kuban DA, Levy LB, Chichakli R, Pollack A, Lee AK, et al. Prostate specific antigen bounce phenomenon after external beam radiation for clinically localized prostate cancer. J Urol 2002;168:2001–5.
- Oesterling JE, Chan DW, Epstein JI, Kimball AW, Jr., Bruzek DJ, Rock RC, et al. Prostate specific antigen in the preoperative and postoperative evaluation of localized prostatic cancer treated with radical prostatectomy. J Urol 1988;139:766–72.
