Abstract
Background. Primary central nervous system lymphoma (PCNSL) is a rare brain tumour with a dismal prognosis. Several phase II studies with high-dose methotrexate-based regimens have shown promising early results, but in all hospital-based data published so far, the disease outcome is poor.
Material and methods. We performed a hospital-based retrospective analysis to evaluate the long-term results of the Nordic type of Bonn chemotherapy regimen in PCNSL patients. The study included 54 patients with newly diagnosed PCNSL who received chemotherapy with curative intent as their first-line treatment.
Results. We found promising response rates, 76% of the patients achieving CR and 22% patients achieving PR, with corresponding two-year EFS 53% and OS 76%. However, with longer follow-up a constant pattern of relapses was observed with only one patient remaining in primary remission after 60 months.
Discussion. The finding suggests that basic biological differences exist between PCNSL and systemic diffuse large B-cell lymphoma and there is a need for consolidation or maintenance therapy after achieving a remission in patients with PCNSL.
Primary central nervous system lymphoma (PCNSL) is a rare brain tumour with an increasing incidence [Citation1]. The standard therapy is a methotrexate (MTX)-containing combination chemotherapy and complete clinical response can be achieved in 30–87% of patients. Addition of high-dose cytarabine to MTX seems to increase both response rate and response duration [Citation2]. Several phase II studies have demonstrated promising disease-free survival data, but in general the number of patients in these trials has been small and the results have been reported after a short follow-up period. Among the best are the results published with the so-called Bonn multiagent chemotherapy regimen. This study reported an 80% two-year overall survival rate in younger patients [Citation3]. In contrast to these promising results in clinical trials, all registry-based analyses published so far have illustrated a dismal outcome in this patient population and found only rare long-term survivors possessing a clear discrepancy with the data extracted from the clinical trials [Citation1,Citation4]. The aim of the present study was to evaluate retrospectively the long-term treatment results achieved with the original or a modified Bonn, the so-called Nordic regimen, in a hospital-based setting.
Material and methods
Patients, staging and treatment
Patients were selected into this study if they had a newly diagnosed PCNSL and were treated with Bonn type multiagent chemotherapy with curative intent at the University Hospitals of Oulu, Helsinki, Tampere and Kuopio and Jyväskylä Central Hospital. The regimen was either the original Bonn regimen as previously published [Citation3] or a Nordic modification of the Bonn regimen. In the modified Nordic regimen the MTX infusion time was reduced to three hours, one rituximab infusion was added to the first treatment cycle and the conventional intrathecal treatment was replaced with liposomal cytarabine injections in cycles 1, 2, 4 and 5. The diagnosis was based on either the histological evaluation of tumour biopsy or positive cerebrospinal fluid (CSF) cytology. All the patients were immunocompetent and considered fit enough for a curative treatment attempt according to the treating physician. Staging included a whole-body computed tomography (CT), brain magnetic resonance imaging (MRI), CSF examination and slit lamp examination. WHO and Karnofsky performance scores were determined for all of the patients. The number of patients treated with the Bonn regimen and the number of patients treated with the modified Bonn regimen was 31 and 23 respectively. Fifteen patients also received four weekly cycles of rituximab at the beginning of the therapy. Six (11.1%) of patients presenting with ocular manifestation also received intravitreal MTX injections. Patient demographics are presented in .
Table I. Patient characteristics.
All of the data was collected retrospectively from clinical records.
Statistics
Survival analyses with corresponding p-values were calculated using the Kaplan-Meier method with the log-rank test. Event-free survival was calculated from the date of diagnosis to either disease progression, failure to achieve complete response, or change of therapy modality due to toxicity or poor response. Overall survival was calculated from the date of diagnosis to the date of death for any reason.
Ethics
The study has an approval from the Oulu University Hospital ethical committee. A permit for patient name registry was applied for and granted for this study.
Response evaluation
The response was evaluated with MRI scans in accordance with the guidelines published by the international PCNSL collaborative group [Citation5].
Salvage therapy
Three patients received first-line salvage autologous stem cell transplantation (ASCT) and six patients whole-brain irradiation due to less than complete response after chemotherapy. Twelve patients received blood-brain barrier disruption therapy in relapse. Five patients entering second CR after salvage chemotherapy received ASCT in relapse.
Results
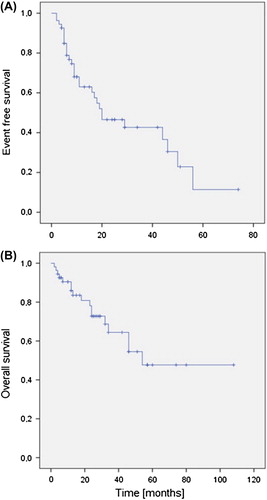
Forty-one (76%) of patients achieved CR and 12 (22%) patients PR, with an overall response rate of 98%. One patient progressed early. One (2%) patient died due to treatment-related adverse events. After a median follow-up period of 54 months, median EFS for whole-study population was 20 months and OS was 54 months (). Two-year EFS and OS rates were 53% and 76% respectively and five-year OS was 38%. There was no statistically significant difference in survival according to MSKC or IELSG score or age under or over 60. Neither were there significant EFS or OS rate differences according to therapy with the original or modified Bonn regimen or according to adding rituximab in the therapy regimen.
Discussion
This is a hospital-based retrospective analysis of 54 PCNSL patients evaluating the results achieved with the Bonn chemotherapy regimen outside clinical trials. In contrast to the situation in systemic DLBCL, where most of the relapses occur during the first two years after the initial therapy, we found a continuous pattern of relapses. In fact only one patient was in primary remission 60 months after the diagnosis.
PCNSL poses a continuous challenge to the treating physician. The standard therapy includes MTX containing polychemotherapy. The benefit of adding cytarabine has been shown in a randomised study by IELSG [Citation2]. Also the study by the German Lymphoma Group suggested a benefit from adding ifosfamide to the induction therapy compared to MTX containing chemotherapy alone, but this was not a randomised comparison [Citation6]. However, no international consensus exists about the optimal combination of the chemotherapy regimen in patients with PCNSL.
In 2003, Pels et al. [Citation3] reported results from their pilot and phase II study with a multiagent chemotherapy, the so-called Bonn regimen, in PCNSL. In their study, the median TTF and OS were 21 and 50 months, with estimated two- and five-year survival rates of 69% and 43% respectively. They also found a drastic difference in survival and TTF of patients under and over 61 years. The younger age group had two- and five-year estimated survival rates of 80% and 75%, compared to over 60-year-old patients, who had two- and five-year estimated survival rates of 43% and 19%, respectively [Citation3]. However, in their second phase II trial of the Bonn type chemotherapy regimen, Pels et al. [Citation7] found a median time to treatment failure of only 8 months.
In our retrospective analysis, two- and five-year estimated OS rates were 76% and 38%, respectively. However, we were not able to demonstrate the drastic outcome difference in different age groups. This may in part be linked to the retrospective nature of the study, reflecting the selection of fitter elderly patients to therapy with curative intent.
Despite the promising response rates, our study demonstrates a high number and continuous pattern of relapses with a longer follow-up time. This highlights that, in PCNSL, it is possible to achieve good responses, but their maintenance is the main problem, indicating that there is an unmet need for effective consolidation and/or maintenance therapies.
Consolidative whole-brain irradiation is sometimes used after chemotherapy. However, radiation therapy increases the risk of late neurotoxicity and probably does not prolong survival [Citation6]. However, in the earlier study on 30 primary CNS lymphoma patients, the reduced-dose WBRT was associated to excellent disease control without neurocognitive decline [Citation8]. The International Extranodal Lymphoma Study Group (IELSG) and Radiation Oncology Therapy Group (RTOG) are currently evaluating the impact of low-dose consolidative radiotherapy (RTOG 1114 phase II randomised trial, NCT01399372).
Another tempting option for consolidation after induction therapy is high-dose therapy and autologous haematopoietic stem cell transplantation. After successful experiences of treating patients with relapsed systemic large B-cell lymphoma with ASCT, this treatment modality has been tested in patients with relapsed intraocular lymphoma and also in recurrent PCNSL. Several studies have shown promising early results [Citation9–13]. At the moment there are two ongoing randomised trials (NCT01011920 and NCT00863460) which compare HD-MTX followed by ASCT or WBRT as a consolidation, thus evaluating the real impact of HDC/ASCT options in comparison to low-dose WBRT.
We found some long-lasting second remission in relapsing patients. Most of these patients had undergone ASCT. The observation underlines the importance of studying its role in PCNSL, both as a front-line therapy and in relapse.
There are several phase II trials showing promising response rates and early relapse-free survival in PCNSL. However, most of these trials lack long-term survival data. The exception is the data published by the BBBD consortium, having a maximal follow-up of 20 years [Citation14]. In contrast to these promising phase II data, all registry-based analyses published so far point out that PCNSL has a dismal prognosis and no, or very few, long-term survivors exists, establishing a clear discrepancy to the positive trial outcomes [Citation1,Citation4]. In the present study we found a constant pattern of late relapses years after achieving a complete clinical response, which is in contrast to the situation in patients presenting with systemic DLBCL. These two entities share a common histology but there is an increasing body of evidence pointing out important differences in their biology and, as we found, also in their relapse pattern. This phenomenon of late relapses has also been shown by Neuwelt et al. [Citation14].
In the present study, we included patients treated with the original Bonn regimen and the Nordic regimen. We reported these patients together because we consider both regimens to be similar enough. Moreover, when analysed according to regimen used, no differences were discovered. 23 patients received one course of rituximab and 15 patients received four treatment doses. These two groups of patients did not have statistically different survival. Rituximab has had a major impact on the treatment results of aggressive systemic B-cell lymphomas. As PCNSL almost always express CD20, it could be anticipated that rituximab would be an important agent. This is further supported by the work of Fritsch et al. [Citation15]. In the Nordic regimen the drug was given only once, which probably is too little, because the drug levels achieved in the circulation after only one course are low.
Our study has two important implications. It seems that intravenous high-dose MTX-based chemotherapy is effective but rarely curative as the sole treatment, and other therapy options should be sought to effectively consolidate the response. The two ongoing randomised studies will give more reliable data from the optimal consolidation method in the future. Of other therapy methods, intra-arterial chemotherapy with blood-brain barrier disruption seems to be one option with a possibility for long-term disease control [Citation14]. Also, new drugs, e.g. pemetrexed, ibrutinib or temozolomide maintenance may change the scenario in the future. The other important conclusion underlines the basic biological differences between PCNSL and systemic DLBCL. This implies the need for long follow-up periods before drawing definitive conclusions from PCNSL clinical trials and the importance of studying the basic biology of this particular disease.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Haldorsen IS, Krossnes BK, Scheie D, Johanssen TB, Mella O, Espeland A. Increasing incidence and continued dismal outcome of primary central nervous system lymphoma in Norway 1989–2003. Cancer 2007;110:1803–14.
- Ferreri AJM, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009;374:1512–20.
- Pels H, Schmidt-Wolf IG, Glasmacher A, Schulz H, Engert A, Diehl V, et al. Primary central nervous system lymphoma: Results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol 2003;21:4489–95.
- Olson JE, Janney CA, Rao RD, Cerhan JR, Kurtin PJ, Schiff D, et al. The continuing increase in the incidence of primary central nervous system non-Hodgkin lymphoma: A surveillance, epidemiology, and end results analysis. Cancer 2002;95:1504–10.
- Abrey LE, Batchelor TT, Ferreri AJ, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol 2005; 23:5034–43.
- Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol 2010;11:1036–47.
- Pels H, Juergens A, Glasmacher A, Schulz H, Engert A, Linnebank M, et al. Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: Results of a phase II study. J Neuro Oncol 2009;91: 299–305.
- Shah GD, Yahalom J, Correa DD, Lai RK, Raizer JJ, Schiff D, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol 2007;25:4730–5.
- Soussain C, Suzan F, Hoang-Xuan K, Cassoux N, Levy V, Azar N, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol 2001;19:742–9.
- Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol 2008;26:2512–8.
- Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica 2008;93:147–8.
- Ferreri AJ, Marturano E. Primary CNS lymphoma. Best Pract Res Clin Haematol 2012;25:119–30.
- Schorb E, Kasenda B, Atta J, Kaun S, Morgner A, Hess G, et al. Prognosis of patients with primary central nervous system lymphoma after high-dose chemotherapy followed by autologous stem cell transplantation. Haematologica 2013; 98:765–70.
- Angelov L, Doolittle ND, Kraemer DF, Siegal T, Barnett GH, Peereboom DM, et al. Blood-brain barrier disruption and intra-arterial methotrexate-based therapy for newly diagnosed primary CNS lymphoma: A multi-institutional experience. J Clin Oncol 2009;27:3503–9.
- Fritsch K, Kasenda B, Hader C, Nikkhah G, Prinz M, Haug V, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol 2011;22:2080–5.