To the Editor,
The use of adjuvant chemotherapy and endocrine therapy for early stage breast cancer has substantially increased. The presentation of the Early Breast Cancer Trialists Collaborative Group (EBCTCG) meta-analyses of adjuvant systemic treatment effectiveness, late 1990s, led to a paradigm shift where adjuvant systemic treatment was no longer reserved for patients with (locally) advanced disease, but also became available to node negative patients [Citation1]. New insights in prognostic factors and improvements in treatment have led to further easing of the eligibility criteria for adjuvant systemic treatment over time. For example, according to the American National Comprehensive Cancer Network (NCCN) breast cancer guidelines, some form of adjuvant systemic treatment could be considered for all breast cancer patients with invasive ductal or lobular tumors larger than 0.5 cm. If a patient has Her2-positive disease, adjuvant systemic treatment could also be considered for tumors smaller than 0.5 cm [Citation2]. Going by these NCCN and other (inter)national guidelines, a proportion of early stage breast cancer patients with a clinical indication for adjuvant systemic treatment have a potential overall survival benefit of as little as 1% – conversely, 99% of these patients potentially only experience side effects and no survival gain. With the exception of patient subgroups deemed at high risk of recurrence and breast cancer mortality (e.g. Her2-positive patients or those 40 years or younger at diagnosis), the general rule of thumb applied in the Netherlands is that adjuvant systemic treatment is advised if treatment reduces the patient's risk of breast cancer death by at least 4% (absolute). This easing of the eligibility criteria for adjuvant systemic treatment is also reflected in the substantial increase in its use in Dutch clinical practice from 1990–2011. Whereas between 1990 and 1997 only 37% of early stage breast cancer patients received adjuvant systemic therapy, in 2011 an average of 70% of early stage breast patients received adjuvant systemic treatment [Citation3,Citation4]. In 2000, just after the publication of the first EBCTCG meta-analysis, a survey amongst Dutch oncologists reported that the majority felt that adjuvant chemotherapy should minimally yield 6–10% overall survival benefit to make it worthwhile for patients with node negative disease [Citation5]. To date no studies have assessed what survival benefit makes endocrine treatment worthwhile according to oncologists. Yet, endocrine treatment duration has been extended more and more (from 2.5 to 5 years and an extension to 10 years is currently topic of debate), whilst studies show non-adherence and/or premature discontinuation of treatment of as much as 40% [Citation6–8].
It has been over a decade since Stiggelbout et al. conducted their survey of oncologists’ views on the survival benefit that makes adjuvant chemotherapy treatment worthwhile. As patients are increasingly diagnosed at earlier stages the benefits adjuvant chemotherapy and endocrine therapy can yield are often small, whereas the potential for side effects remains undiminished [Citation4]. In view of the substantial increase in adjuvant systemic treatment use in the past decades, we replicated our study assessing how much treatment benefit, given the potential side effects, Dutch oncologists require to tip the scale in favor of adjuvant systemic treatment.
Methods
Recruitment of participants
This study was conducted as part of a larger project investigating oncologists’ views on risk prediction models and their use in clinical practice to guide adjuvant systemic treatment decisions [Citation9]. Medical and surgical oncologists were eligible to participate in the current study. The Comprehensive Cancer Center the Netherlands (IKNL) sent out an invitation to complete the anonymous online survey on our behalf to the members of all the medical oncology and breast cancer working parties. IKNL has a nationwide coverage, facilitating the recruitment of our target. A reminder was sent four weeks later.
Measures and data analyses
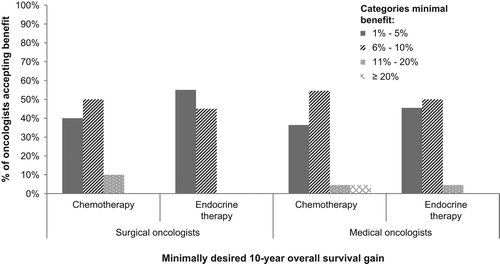
To determine the minimal adjuvant systemic treatment 10-years overall survival benefit participants deemed sufficient, they were asked: “What is the minimal percentage treatment benefit that in your opinion makes treatment X worthwhile, given the side-effects?”. This was a multiple choice question, where participants could choose from the following categories: “1–5%”, “6–10%”, “11–20%” or “more than 20%”. If they indicated that the treatment benefit they required was between 1% and 5%, they were asked to provide us with the exact percentage. We also assessed some background characteristics, such as age, type of hospital they work at and level of experience. All analyses were performed using SPSS 20.
Results
We included 42 oncologists, of who half were surgeons. Participants were 49 years on average (range 31–64 years), 58% were male and 80% worked in a teaching hospital (general or academic) (). For privacy reasons we could not access data on the size and composition of the IKNL working parties approached for this study; hence, we are unable to estimate our response rate.
Table I. Oncologists’ characteristics [N (%)].
Chemotherapy
Half of surgical and medical oncologists indicated that between 6% and 10% survival gain is the minimal percentage benefit that offsets the potential side effects due to treatment (). Of the 16 (38%) oncologists who indicated that 1–5% was sufficient survival gain, the minimally required benefit ranged from 3% (N = 2, 13%) to 5% (N = 9, 56%).
Endocrine therapy
Medical oncologists tended to require greater survival benefits from endocrine therapy than surgical oncologists, but the difference was not statistically significant (). If oncologists (N = 21, 50%) thought that 1–5% overall survival benefit was sufficient to justify endocrine treatment, the minimally required benefit threshold ranged from 3% (N = 9, 43%) to 5% (N = 7, 24%).
Generally, younger (< 50 years) and female oncologists more often indicated that a minimal overall survival benefit (1–5%) was sufficient to offset potential treatment side effects for both chemotherapy and endocrine therapy (data not shown; differences not statistically significant).
Discussion
There was a wide range in the benefit required from adjuvant systemic treatment within both surgical and medical oncologists. Most oncologists required 6–10% survival benefit to recommend adjuvant chemotherapy. When it came to endocrine therapy most surgical oncologists had a lower required benefit threshold (1–5%) compared to medical oncologists (6–10%).
The current study is the first to explore oncologists’ minimally desired treatment benefit of endocrine therapy. Although a larger proportion of medical compared to surgical oncologists require an overall survival benefit equal to that of chemotherapy, half of our respondents required less treatment benefit to justify endocrine treatment which suggests that there is a tendency to underestimate the impact of endocrine therapy. Although often perceived as less aggressive, there is substantial non-adherence to endocrine therapy, moreover, although side effects may be less severe, treatment lasts for a substantially longer time. A recent study showed that overall, patients consider the efficacy of treatment to be the most important factor, but it was closely followed by side effects joint and muscle pain and risk of endometrial cancer. About one in six patients even felt that the treatment benefits did not outweigh the side effects [Citation10]. This illustrates the importance of taking patients’ values into account when deciding about treatment.
Interestingly, even though the eligibility criteria for adjuvant systemic treatment have become broader, oncologists’ minimally required benefit from adjuvant chemotherapy remains unchanged compared to the findings reported by Stiggelbout et al. well over a decade ago. Perhaps this lack of change, is a sign of the overriding sense that by casting such a wide net, i.e. having such broad guidelines, more harm is done than good, as the vast majority (> 60%) of patients currently undergoing adjuvant systemic treatment, probably do not need it [Citation11]. Unfortunately, the currently available tools are not yet sensitive enough to help clinicians determine which patients can forego treatment, without negatively affecting their (recurrence-free) survival [Citation12].
Regrettably, our sample is small; nonetheless, our findings indicate that for both chemotherapy and endocrine therapy, most oncologists agree that treatment is worthwhile if the potential survival benefit is 10% or more. There only seems to be a difference of opinion if the potential benefit is less than 10%. Oncologists that participated in the current study require greater survival benefits from adjuvant systemic treatment, than the threshold indicated in the Dutch breast cancer guideline (i.e. ≥ 4%), to deem treatment worthwhile. Over the past decades patients are diagnosed at earlier stages and have a good prognosis a priori. The fact that during the same period the use of adjuvant systemic treatment has virtually doubled, suggests that oncologists do not adhere to their own minimally desired treatment benefit when recommending treatment to patients. This stresses the imperative for oncologists and patients to critically mull over whether the potential treatment benefits are worthwhile in light of the side effects associated with treatment. Especially when the potential treatment benefit is small, patient preferences could be the overriding factor when deciding about treatment. However, to make this possible, patients should be adequately informed about all the relevant treatment options (including forgoing treatment), their potential benefits and (main) side effects, and afforded the opportunity to freely discuss their thoughts, concerns and any doubts about treatment with their oncologist. Such an open exchange of information (oncologist) and considerations (patients) could help patients and their oncologists to decide on the best course of action with which both parties feel comfortable.
Acknowledgments
We would like to thank the participating oncologists for taking the time to complete our survey. Further, we are grateful to the Comprehensive Cancer Centers, The Netherlands and Ms. Cora Bakker for their help in disseminating our survey. E. G. Engelhardt is funded by a grant from the Dutch Cancer Society (grant UL2010-4805).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Early Breast Cancer Trialists Collaborative Group (EBCTCG). Polychemotherapy for early breast cancer: An overview of the randomised trials. Lancet 1998;352: 930–42.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): Breast cancer version1.2014. 01-01-2014.
- Sukel M, van de Poll-Franse L, Nieuwenhuijzen G, Vreugdenhil G, Herings R, Coebergh J, et al. Substantial increase in the use of adjuvant systemic treatment for early stage breast cancer reflects changes in guidelines in the period 1990–2006 in the southeastern Netherlands. Eur J Cancer 2008;44:1846–54.
- Dutch Cancer Society and Comprehensive Cancer Centers, The Netherlands. Cijfers over borstkanker. 2013. 3-6-2014.
- Stiggelbout AM, de Haes JCJM, van de Velde CJH. Adjuvant chemotherapy in node negative breast cancer: Patterns of use and oncologists’ preferences. Ann Oncol 2000;11:631–3.
- Fink AK, Gurwitz J, Rakowski W, Guadagnoli E, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor positive breast cancer. J Clin Oncol 2004;22:3309–15.
- Lash TL, Fox MP, Westrup JL, Fink AK, Silliman RA. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat 2006;99:215–20.
- Partridge AH, LaFountain A, Mayer E, Taylor BS, Winer E, Asnis-Alibozek A. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol 2008;26:556–62.
- Engelhardt E, Pieterse A, an Duijn-Bakker N, Kroep J, de Haes J, Smets E, et al. Breast cancer specialists’ views on and use of risk prediction models in clinical practice: A mixed methods approach. Acta Oncol Epub 2014 Oct 2013.
- Wouters H, Maatman GA, Van Dijk L, Bouvy ML, Vree R, Van Geffen ECG, et al. Trade-off preferences regarding adjuvant endocrine therapy among women with estrogen receptor-positive breast cancer. Ann Oncol 2013;24:2324–9.
- Schmidt M, Victor A, Bratzel D, Boehm D, Cotarelo C, Lebrecht A, et al. Long-term outcome prediction by clinicopathological risk classification algorithms in node-negative breast cancer: Comparison between Adjuvant!, St Gallen, and a novel risk algorithm used in the prospective randomized Node-Negative-Breast Cancer-3 (NNBC-3) trial. Ann Oncol 2009;20:258–64.
- Engelhardt EG, Garvelink MM, de Haes JH, van der Hoeven JJ, Smets EM, Pieterse AH, et al. Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: A systematic review of risk prediction models. J Clin Oncol 2014;32:238–50.