Abstract
Background. Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous group of B-cell lymphomas. Five clinical adverse risk factors are merged into the International Prognostic Index (IPI), which is the major tool for prognostication. In contrast to Hodgkin's lymphoma, gender is not considered as an adverse risk factor for DLBCL patients. As we clinically had observed a very good survival rate in young female patients we hypothesised that there was a gender difference in survival due to the hormonal status of the patient.
Material and methods. We conducted a registry-based retrospective cohort study of all Swedish DLBCL patients diagnosed between 2000 and 2013, to evaluate the impact of gender for survival from DLBCL.
Results. In total, 7166 patients were included for further analysis. No survival difference was found between the genders when the entire population was analysed. However, analysis of 880 young patients of pre-menopausal age (i.e. 52 years) revealed that women had a longer survival compared to men of the same age group (p = 0.007). This was not found for patients older than menopausal age. In a relative survival multifactorial model adjusted for stage, ECOG performance status, serum lactate dehydrogenase and two or more extranodal sites, male gender was found to be an adverse risk factor for patients younger than 52 years (RR 1.51, 95% CI 1.14–1.88), but not for older patients (RR 0.99, 95% CI 0.89–1.10).
Conclusion. This is one of the largest population-based studies of DLBCL presented to date. Most interestingly, we found male gender to be a significant adverse risk factor compared to fertile women whereas we found no survival differences between genders in the older sub-population.
The majority of diffuse large B-cell lymphoma (DLBCL) are diagnosed at an age older than 60 years [Citation1], in contrast to Hodgkin's lymphoma which predominantly affects a younger population [Citation2]. Besides this, both survival and prognostic factors differ between the diseases. For DLBCL, the International Prognostic Index (IPI) is the most important clinical tool for determining prognosis [Citation3]. The IPI incorporates five adverse risk factors: age above 60 years; Ann Arbor stage III or IV; elevated serum lactate dehydrogenase (S-LDH); Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher; and involvement of two or more extranodal sites [Citation4]. However, the IPI was calculated 20 years ago from a cohort where standard treatment was a combination-chemotherapy including doxorubicin with reported survival rates of about 50%. In the beginning of the third millennium the monoclonal antibody rituximab, directed against CD20, was added to the treatment regime, whereafter survival rates of 70% have been reported in an elderly population [Citation5] and even higher rates in a younger population [Citation6]. IPI was recently reported to remain as a functional prognostic tool for R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) treated patients [Citation3].
For Hodgkin's lymphoma, other clinical risk factors are used for prognostication and determination of treatment strategies [Citation7]. One adverse risk factor for Hodgkin's lymphoma is male gender. In contrast, studies of DLBCL have not always have found a significant survival difference between the genders [Citation8], although some studies have [Citation9,Citation10] and the clinical importance could thus be debated [Citation11]. However, many studies have addressed an older population of patients with DLBCL [Citation12–14] whereas fewer exclusively address a younger DLBCL population [Citation6].
One major gender difference between a younger and an older population are the levels of female sex hormones in young women. Female sex hormone levels decrease dramatically after menopause and which makes the differences between the genders less distinguishable. Oestrogen is one of the main female sex hormones but also affects the immune system and might play an important role for malignancies [Citation15]. Fertile female dogs have been found to have a lower risk of developing lymphoma compared to spayed female and male dogs [Citation16], and in humans, surgical menopause increases the risk for B-cell lymphoma [Citation17]. Taken together with our clinical observations that the younger DLBCL population fared better, we hypothesised a potential difference in outcome between genders may be due to female sex hormones and consequently only detectable in a younger population. Therefore, we conducted a retrospective population-based cohort study of DLBCL patients with the aim of specifically addressing and describing gender differences.
Patients and methods
Lymphoma Registry
The Swedish Cancer Registry was founded in 1958 and, on a compulsory basis, pathologists report all malignant pathology specimens and the responsible physician reports all patients with newly-diagnosed cancer. The Swedish Lymphoma Registry (SLR) was initiated by the Swedish Lymphoma Group in the year 2000, as a sub-registry of the Swedish Cancer Registry. Each one of the six health care regions in Sweden scan the Swedish Cancer Registry and collect additional information about all lymphomas from the responsible physician. The information is then sent unidentified to the SLR.
The SLR contains information about diagnosis, gender, age at diagnosis, as well as certain clinical characteristics such as stage and IPI. Since 1 January 2007, the SLR also contains information about first-line treatment. However, the registry does not contain information about treatment outcome for the entire study period, comorbidities and cause of death. Nor are children below the age of 16 and persons aged 16–19 who are treated at a paediatric ward included in the registry. Incidentally found cases, where diagnosis was determined at autopsy, are also not included. Compared to the compulsory Swedish Cancer Registry, the SLR covers approximately 95–97% of all lymphoma cases in Sweden [Citation11].
Study population
This retrospective study is based on a population cohort that includes all patients diagnosed with DLBCL-NOS and diffuse non-Hodgkin B-cell lymphoma registered in the SLR between 1 January 2000 and 1 November 2013. The SLR classifies lymphomas according to ICD-O3 and no further sub-classification is included in the registry. Patients diagnosed at an age younger than 20 years (n = 13) were omitted due to incomplete registry in this age group, as described above. Data collected were: year of diagnosis, age, gender, Ann Arbor stage, ECOG performance status, extranodal sites, S-LDH, IPI, age adjusted IPI (aaIPI). Reliable data for diagnosis was missing in 28 cases, which were excluded from further analysis, leaving 7166 patients in the study. Data on survival were obtained from the Swedish Population Registry, as of 4 December 2013.
Ethics
This study was approved by the local ethical review board at Uppsala University, which waived written consent due to the fact that all data retrieved from the Swedish Lymphoma Registry was anonymised. The study was conducted in accordance with the rules of the Helsinki Declaration.
Statistics
Overall survival was calculated from date of diagnosis until last follow-up or death. Survival curves were estimated according to the Kaplan-Meier method and associated log rank tests were used to examine survival disparities between different cohorts of the study population.
Relative survival (RS) analysis was used as an additional method to measure survival from DLBCL. In contrast to overall survival, which takes into consideration all deaths regardless of cause and gives a crude measurement of survival, RS is a commonly used method for capturing net survival in population-based cancer studies, where it computes mortality directly or indirectly correlated to the disease without requiring data about actual cause of death [Citation18]. The definition of RS can be expressed as a ratio between observed survival in the study population and the expected survival in a comparable group from the general population. Data regarding the reference population was extracted from the database www.mortality.org.
A RS regression model was used, according to the maximum likelihood method, and expressed as a risk ratio (RR), to evaluate the prognostic impact of clinical risk factors [Citation19]. Testing of equal means was performed with a t-test (two-sided). The testing for equal proportions was performed with χ2. All statistical analyses were performed using the R statistical program version 2.14.2 (www.r-project.org). Probabilities with a p-value < 0.05 were considered statistically significant.
Results
In total 7166 patients with DLBCL were identified in the SLR between 1 January 2000 and 1 November 2013. There was a slightly lower proportion of women (n = 3223, 45%) than men (n = 3943, 55%) with DLBCL. The median follow-up time of surviving patients was 5.3 years.
The mean age for all patients was 68 years (range 20–105), with women diagnosed at a slightly but significantly higher age (mean age 69 years, range 20–105) compared to the men (mean age 67 years, range 20–99) (p < 0.0001). There was no significant difference in the observed overall survival between genders (p = 0.55) (). However, when patients younger than 52 years, which is the mean age of menopause in Sweden [Citation20], were analysed separately, women had a significantly longer overall survival compared to men (p = 0.007), whereas no survival difference between the genders was observed in the age group of 52 years or older (p = 0.4) (). To adjust for any survival differences due to gender related background mortality, RS analysis was performed, but resulted in similar curves (). To reduce the probability that the survival difference between genders were due to only age, we further subdivided the entire population into three age categories; young patients aged younger than 52 years, middle-aged patients aged 52–60 years, and patients older than 60 years (). Log rank test for observed overall survival found no survival difference between women and men in the middle-aged patient group (p = 0.4) nor in the old patient group (older than 60 years) (p = 0.7).
Figure 1. Survival of DLBCL diagnosed in Sweden 2000–2013. (A) Observed overall survival for all men and women (log rank test p = 0.6). (B) Observed overall survival for young and old men and women (log rank test for young persons p = 0.007 and for old persons p = 0.4). Cut-off for young versus old is 51–52 years (mean age for menopause for women in Sweden). (C) Relative survival for young and old men and women. (D) Relative survival for patients younger than 52 years, middle aged patients aged 52–60 years, and patients older than 60 years.
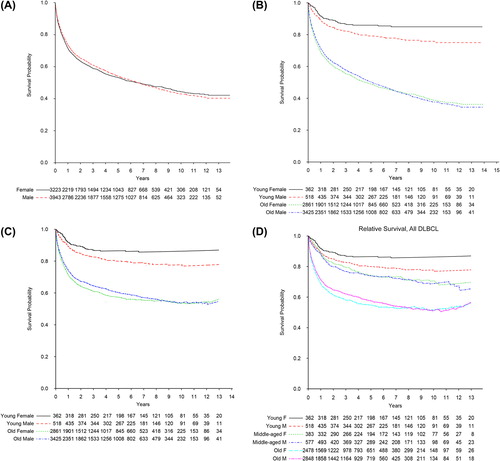
In order to further study the survival differences between male and female patients, the factors included in IPI were studied in more detail to specifically detect differences in clinical presentation. Clinical characteristics are summarised in . For the entire population, men had a significantly higher proportion of high Ann Arbor stage (p = 0.015), but a lower proportion of high ECOG performance status cases (p < 0.0001) and a tendency towards a significantly higher proportion with normal S-LDH (p = 0.073), compared to women. However, when the analysis was stratified according to age younger or older than 51 years, the differences between genders were limited to patients older than 51 years. For patients younger than 51 years, there were no differences between genders regarding proportions of Ann Arbor stage, elevated S-LDH, ECOG performance status, aaIPI and IPI. However, young patients of male sex had a small but significantly higher proportion with extranodal disease compared to female patients 51 years or younger.
Table I. Clinical characteristics of DLBCL in Sweden 2000–2013.
In order to further analyse the impact of sex as an independent factor for survival, RS RRs were analysed in a multifactorial model, including the adverse risk factors normally included in IPI. Age was omitted from the analysis since the cut-off as an adverse risk factor in IPI is 60 years. Two analyses were made, one with each risk factor analysed in full, and one where ECOG performance status and Ann Arbor stage were grouped according to risk given in the IPI. summarises the results from the RS RR analysis.
Table II. Relative risk ratio (RR) of DLBCL mortality in Sweden 2000–2013.
When the entire population was analysed, sex was not found to be significantly correlated with adverse outcome. Neither was Ann Arbor stage II and III, as well as extranodal involvement of two or more sites, correlated to worse outcome. However, Ann Arbor stage IV was found to have a significantly adverse RR of 1.34 (95% CI 1.16–1.52, p = 0.001) together with high S-LDH (RR 1.60, 95% CI 1.47–1.73, p < 0.001) and performance status 1–4 (RR 3.09; 5.95; 9.44; 16.0 with 95% CI 2.92–3.27; 5.75–6.14; 9.24–9.63; 15.8–16.2, respectively, p < 0.001).
When patients older than 52 years were analysed separately, the same risk factors remained significant (see ). However, when patients aged 51 years or younger were analysed separately, elevated S-LDH and performance status 1–4 remained with significantly worse RR, whereas Ann Arbor stage IV no longer was significantly correlated with worse outcome (RR 1.37, 95% CI 0.77–1.97, p = 0.3). Most interestingly, male gender was found to significantly correlate with worse outcome in the younger population (RR 1.51, 95% CI 1.14–1.88, p = 0.029).
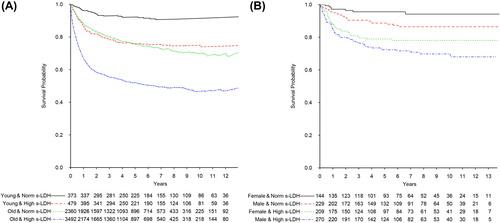
Further analysis of RS revealed a decline in survival for each consecutive higher stage for the entire population (, ). This was in contrast to patients 51 years or younger where there was almost no difference in survival for stages 1–3 and the difference for stage IV never reached significance (p = 0.3) (, ). Furthermore, S-LDH had a prognostic impact in both the young and the old age group (RR 2.02; 1.59, 95% CI 1.53–2.52; 1.45–1.72, respectively) (). For young patients up to age 52, women with normal S-LDH had a significantly longer overall survival compared to men with normal S-LDH (p = 0.025) whereas there was no significant overall survival difference between young women and men with elevated S-LDH (p = 0.092) ().
Figure 2. Relative survival of DLBCL in Sweden diagnosed 2000–2013 according to Ann Arbor stage. (A) All patients grouped according to Ann Arbor stage. (B) Patients younger than 52 years (mean age for menopause for women in Sweden) according to gender and Ann Arbor stage.
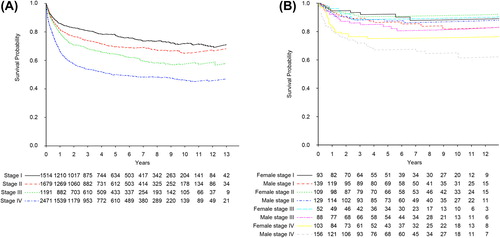
Figure 3. (A) Relative survival of all DLBCL patients, and (B) observed overall survival of DLBCL patients younger than 52 years, diagnosed in Sweden 2000–2013. (A) Patients grouped according to normal (Norm) or high serum lactate dehydrogenase (S-LDH) and age. Cut-off for young versus old is 51–52 years (mean age for menopause for women in Sweden). (B) Patients younger than 52 years grouped according to normal (Norm) or high serum lactate dehydrogenase (S-LDH) and gender. Women with normal S-LDH had a significantly longer overall survival compared to the men (log rank test p = 0.03) whereas no difference was found for patients with elevated S-LDH (log rank test p = 0.09).
With worse ECOG performance status, there was a decline in overall survival for the entire population (data not shown). However, there were no significant differences between the genders that could explain the better survival in female patients.
Interestingly, there were similar proportions of patients with two or more extranodal locations of the disease in both the young (51 years or younger) and old (52 years or older) patient groups.
Discussion
This retrospective population-based study is, to our knowledge, one of the largest population-based studies of DLBCL published so far [Citation11,Citation21,Citation22]. Here we specifically address the impact of gender upon prognosis of DLBCL. The size of the study enables us to perform a thorough evaluation of the characteristics of DLBCL subgroups.
In line with previous results that have found a predominance of males among lymphoma patients with lymphomas other than Hodgkin's [Citation23], we found a higher proportion of males diagnosed with DLBCL in Sweden between 1 January 2000 and 1 November 2013. However, the impact of gender upon prognosis from DLBCL has varied between studies [Citation8,Citation10]. We found no significant survival difference between genders when the entire study population was analysed.
However, we had previously hypothesised a possible gender difference to be due to female sex hormones. Since female sex hormone levels change from fertile age to post-menopausal age [Citation24], we hypothesised the difference to be most distinguishable between fertile women, and men in the same age. Unfortunately, the SLR lacks information regarding menopause status.
In Sweden, the mean age for menopause is about 51 years [Citation20]. Normal menopause is considered to begin from 45 years of age and by 54 most women have undergone menopause [Citation25]. Hence, we considered all patients 51 years old, or younger, of interest for further analysis of potential differences between genders, since most women in this age group had not yet undergone menopause. In this sub-population women had a significantly better RS (p = 0.007), in contrast to the older sub-population (52 years or older) ().
However, a survival difference between genders may depend upon differences in the presentation of the disease DLBCL, with different proportions of adverse risk factors for women and men. Therefore further sub-groupings were performed and analysed. Mean age was the same for both younger groups (up to age 51) of women and men. However, since we lack data about treatment outcome and cause of death, analysis of RS was performed to circumvent differences in background mortality for women and men of the same age. In a recent study on a material from the SLR, the higher survival for women was compensated for by only 2 years extra in age [Citation11]. However, this was an analysis of the entire age range. Although the majority of patients are the same in the aforementioned study and in our study, we limited our analysis to women of fertile age and included a larger material with a longer follow-up period for the earliest diagnosed patients.
One disadvantage of our study are the limitations that follow the specific study design, which does not allow further analysis of the underlying cause of the higher survival rate for young women compared to young men (i.e. 51 years or younger). Differences in clinical presentation and different proportions of known adverse risk factors could partly explain the survival difference between the genders. However, the better survival for our young women persisted in sub-analyses when adjusted for the risk factors S-LDH, stage, ECOG performance status and two or more extranodal sites. In the multivariate analysis we included the adverse risk factors normally included in the IPI. However, age was omitted from our analysis since IPI consider age above 60 years as an adverse risk factor and we limited our analysis of the young sub-population to patients of an age of 51 years or younger. In this multivariate analysis of relative RRs male sex persisted as an adverse risk factor, implying that the survival difference is partly due to gender-specific variables. The SLR is limited in its information about S-LDH levels, which prevent calculation and analysis of an enhanced IPI, which has been described to improve risk stratification [Citation8].
It has been known many years that women and men are differently affected by diseases. It is also known that the response of the immune system differs between women and men. Women have a lower incidence of infections [Citation26] and a higher survival rate from sepsis than men [Citation27]. However, the differences in immune response make women more prone to develop post-infectious immunopathologic events and are at greater risk for developing autoimmune diseases than men [Citation28]. Women are also less susceptible to developing cancer and haematological malignancies, including non-Hodgkin's lymphoma, in particular [Citation23,Citation29]. This could explain the difference in proportions of women and men diagnosed with DLBCL in our study. However, other mechanisms may also contribute to the survival differences found between women and men. For example, in a recently published paper, women had a significantly prolonged elimination half-life for rituximab compared to men [Citation30].
Several factors may cooperate to develop these gender disparities. There are different expression levels of not only sex-linked, but also autosomal, genes between genders [Citation31] as well as differences in antioxidative capacity [Citation29]. Environmental differences between genders could affect cancer incidence and are known for some cancers but not as a general phenomenon [Citation32]. However, several of these factors are persistent throughout life and assert a cumulative gender-specific risk modulating effect. In our analysis, women had a significantly better survival from DLBCL compared to men, but only for the subgroup with fertile women. This indicates that mechanisms that are more pronounced during women's fertile age are involved.
Sex hormone expression differs between genders and is more pronounced between fertile women and men. Both oestrogens and androgens have been found to modulate immune responses, and thus determine gender-specific differences in the disease panorama. Oestrogen has the potential to affect both B- and T-lymphocytes as well as dendritic cells and macrophages. By means of its intracellular receptors, oestrogen can inhibit pro-inflammatory genes as well as modulate the immune response and cytokine production by altering levels of regulatory microRNA [Citation28]. However, after menopause, oestrogen levels diminish and the impact of oestrogen upon immune system becomes less evident. The impact of oestrogen upon lymphomagenesis has been described in dogs, where female dogs had a lower incidence of diagnosis with lymphoma compared to male and spayed female dogs [Citation16]. Furthermore, lymphoma cells have been found to grow faster in male mice and ovariectomised female mice compared to intact female mice [Citation15]. Together with our finding that a gender difference only is present between fertile women and men of the same age these observations suggest the mechanism to be correlated to physiological characteristics in the fertile female body. However, the exact underlying mechanism must be investigated in future studies.
In summary, this study presents one of the largest population-based cohorts of DLBCL patients published so far [Citation11,Citation21,Citation22] with thorough analysis of clinical characteristics with emphasis on gender. The finding of a significant survival advantage for young women which was absent in the older sub-population of women, makes this study especially interesting. To our knowledge, this is the first study that specifically addresses gender differences in a population of women of fertile age in comparison to men of the same age. This enhances previous findings [Citation9,Citation10] that there is indeed a survival advantage for some women, but also raises questions about whether young men could benefit from tailored regimens with more intensive immunotherapy.
Acknowledgments
This work was supported by the County Council of Uppsala, Sweden, and the Lions Cancer Research Foundation, Uppsala University Hospital, Uppsala, Sweden.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Castillo JJ, Winer ES, Olszewski AJ. Sites of extranodal involvement are prognostic in patients with diffuse large B-cell lymphoma in the rituximab era: An analysis of the surveillance, epidemiology and end results database. Am J Hematol 2014;89:310–4.
- Sjoberg J, Halthur C, Kristinsson SY, Landgren O, Nygell UA, Dickman PW, et al. Progress in Hodgkin lymphoma: A population-based study on patients diagnosed in Sweden from 1973–2009. Blood 2012;4:990–6.
- Ziepert M, Hasenclever D, Kuhnt E, Glass B, Schmitz N, Pfreundschuh M, et al. Standard International prognostic index remains a valid predictor of outcome for patients with aggressive CD20 + B-cell lymphoma in the rituximab era. J Clin Oncol 2010;14:2373–80.
- A predictive model for aggressive non-Hodgkin’s lymphoma. The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. N Engl J Med 1993;14:987–94.
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;4:235–42.
- Pfreundschuh M, Kuhnt E, Trumper L, Osterborg A, Trneny M, Shepherd L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good- prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011; 11:1013–22.
- Eichenauer DA, Engert A. Advances in the treatment of Hodgkin lymphoma. Int J Hematol 2000;7:235–40.
- Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42.
- Riihijarvi S, Taskinen M, Jerkeman M, Leppa S. Male gender is an adverse prognostic factor in B-cell lymphoma patients treated with immunochemotherapy. Eur J Haematol 2011;2:124–8.
- Carella AM, de Souza CA, Luminari S, Marcheselli L, Chiappella A, di Rocco A, et al. Prognostic role of gender in diffuse large B-cell lymphoma treated with rituximab containing regimens: A Fondazione Italiana Linfomi/Grupo de Estudos em Molestias Onco-Hematologicas retrospective study. Leuk Lymph 2013;1:53–7.
- Szekely E, Hagberg O, Arnljots K, Jerkeman M. Improvement in survival of diffuse large B-cell lymphoma in relation to age, gender, IPI and extranodal presentation: A population based Swedish Lymphoma Registry study. Leuk Lymph 2014;55:1838–43.
- Varga C, Holcroft C, Kezouh A, Bucatel S, Johnson N, Petrogiannis-Haliotis T, et al. Comparison of outcomes among patients aged 80 and over and younger patients with diffuse large B-cell lymphoma: A population based study. Leuk Lymph 2014;55:533–7.
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010;12:2040–5.
- Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20 + B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol 2008;2:105–16.
- Yakimchuk K, Jondal M, Okret S. Estrogen receptor alpha and beta in the normal immune system and in lymphoid malignancies. Mol Cell Endocrinol 2013;1–2:121–9.
- Villamil JA, Henry CJ, Hahn AW, Bryan JN, Tyler JW, Caldwell CW. Hormonal and sex impact on the epidemiology of canine lymphoma. J Cancer Epidemiol 2009; 2009:591753.
- Lu Y, Wang SS, Sullivan-Halley J, Chang ET, Clarke CA, Henderson KD, et al. Oral contraceptives, menopausal hormone therapy use and risk of B-cell non-Hodgkin lymphoma in the California Teachers Study. Int J Cancer 2011;4:974–82.
- Ederer F, Axtell LM, Cutler SJ. The relative survival rate: A statistical methodology. Natl Cancer Inst Monogr 1961; 6:101–21.
- Pohar M, Stare J. Relative survival analysis in R. Comput Methods Programs Biomed 2006;3:272–8.
- Rodstrom K, Bengtsson C, Lissner L, Bjorkelund C. Reproducibility of self-reported menopause age at the 24-year follow-up of a population study of women in Goteborg, Sweden. Menopause 2005;3:275–80.
- Moller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: Clinical implications of extranodal versus nodal presentation – a population-based study of 1575 cases. Br J Haematol 2004;2:151–9.
- Lee L, Crump M, Khor S, Hoch JS, Luo J, Bremner K, et al. Impact of rituximab on treatment outcomes of patients with diffuse large b-cell lymphoma: A population-based analysis. Br J Haematol 2012;4:481–8.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin 2013;1:11–30.
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005;4:411–23.
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998; 12:1271–6.
- Klein SL. The effects of hormones on sex differences in infection: From genes to behavior. Neurosci Biobehav Rev 2000;6:627–38.
- Drechsler S, Weixelbaumer K, Raeven P, Jafarmadar M, Khadem A, van Griensven M, et al. Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post- traumatic sepsis. PLoS One 2012;12:e51457.
- Klein SL. Immune cells have sex and so should journal articles. Endocrinology 2012;6:2544–50.
- Cook MB, Dawsey SM, Freedman ND, Inskip PD, Wichner SM, Quraishi SM, et al. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev 2009;4:1174–82.
- Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012;14:3276–84.
- Arnold AP, van Nas A, Lusis AJ. Systems biology asks new questions about sex differences. Trends Endocrinol Metab 2009;10:471–6.
- Vineis P, Wild CP. Global cancer patterns: Causes and prevention. Lancet 2014;383:549–57.
