Abstract
Background. The Norwegian Rectal Cancer Project was initated in 1993 with the aims of improving surgery, decreasing local recurrence rates, improving survival, and establishing a national rectal cancer registry. Here we present results from the Norwegian Colorectal Cancer Registry (NCCR) from 1993 to 2010.
Material and methods. A total of 15 193 patients were diagnosed with rectal cancer in Norway 1993–2010, and were registered with clinical data regarding diagnosis, treatment, locoregional recurrences and distant metastases. Of these, 10 796 with non-metastatic disease underwent tumour resection. The results were stratified into five time periods, and the treatment outcomes were compared. Recurrence rates are presented for the 9785 patients who underwent curative major resection (R0/R1).
Results. Among all 15 193 patients, relative five-year survival increased from 54.1% in 1993–1997 to 63.4% in 2007–2010 (p < 0.001). Among the 10 796 patients with stage I–III disease who underwent tumour resection, from 1993–1997 to 2007–2010, relative five-year survival improved from 71.2% to 80.6% (p < 0.001). An increasing proportion of these patients underwent surgery at large-volume hospitals; and 30- and 100-day mortality rates, respectively, decreased from 3.0% to 1.4% (p < 0.001) and from 5.1% to 3.0% (p < 0.011). Use of preoperative chemoradiotherapy increased from 6.5% in 1993 to 39.0% in 2010 (p < 0.001). Estimated local recurrence rate after major resection (R0/R1) decreased from 14.5% in 1993–1997 to 5.0% in 2007–2009 (p < 0.001), and distant recurrence rate decreased from 26.0% to 20.2% (p < 0.001).
Conclusion. Long-term outcomes from a national population-based rectal cancer registry are presented. Improvements in rectal cancer treatment have led to decreased recurrence rates of 5% and increased survival on a national level.
Colorectal cancer is one of the most common cancers worldwide, with the highest incidence in Europe, Australia, and US [Citation1]. Historically, rectal cancer has been associated with high local recurrence rates with severe symptom burden and poor survival. The first main treatment challenge is to reduce local recurrence rates and improve survival without impairing functional outcomes and quality of life. Development of the total mesorectal excision (TME) technique significantly improved outcome [Citation2]. Additionally, there have been substantial improvements in radiology, pathology, and multimodality treatments [Citation3]. The second main challenge is to reduce distant recurrence, and treatment strategies have evolved further and are becoming more complex and tailored [Citation4].
In 1993, the Norwegian Rectal Cancer Project was initiated with the aim of improving nationwide rectal cancer outcomes after curative surgery. The objectives were to improve rectal cancer surgery by introducing TME surgery, reduce local recurrence rates and improve survival, and establish a registry for quality control [Citation5]. The strategy was to establish a national consensus for the management of patients with rectal cancer, and to monitor outcomes and report them back to each institution. For this purpose, the Norwegian Colorectal Cancer Registry (NCCR) was established as the first national population-based registry, including all patients with rectal cancer from 1993, and colon cancer from 2007 [Citation5].
When TME was introduced as the national standard of surgery [Citation2], numerous multidisciplinary workshops were held [Citation5]. Over the following years, diagnosis and treatment methods changed to include assessment of circumferential resection margin (CRM) [Citation6], magnetic resonance imaging (MRI) for locoregional staging [Citation7], computed tomography (CT) for metastases detection, establishment of multidisciplinary teams [Citation8], and a tailored approach to preoperative radiotherapy or chemoradiotherapy [Citation9–12]. Treatment guidelines have evolved based on scientific evidence [Citation4], and the NCCR multidisciplinary board regularly updates the national guidelines.
The present study aimed to evaluate the results of rectal cancer treatment in Norway by reporting the crude outcomes from the NCCR for all patients with rectal cancer who were operated with curative intent from 1993 to 2010. Long-term outcomes of rectal cancer treatment from a national clinical quality registry are reported. Time trends on local recurrence rates, metachronous distant metastases, and survival, as well as important diagnostic and treatment parameters are presented.
Material and methods
Cancer Registry of Norway (CRN)
Established in 1953, the Cancer Registry of Norway (CRN) registers all cancer incidents in Norway by legal obligation. The patients are identified by a unique 11-digit personal number, enabling individual follow-up through linkage with different data sources. Clinicians are required to report all patients diagnosed with primary cancer or recurrence. All pathology reports that include cancer diagnosis are reported separately. Individual patient data are linked with radiotherapy reports, the Norwegian Patient Register (patients’ records from public hospitals), and the Norwegian Cause of Death Registry. A systematic quality control regimen ensures that reminders are sent for missing data. The CRN has been proven to have a very high degree of completeness (98.8%) and timeliness for registering new cases [Citation13].
Norwegian Colorectal Cancer Registry (NCCR)
Established within the CRN in 1993, the Norwegian Rectal Cancer Registry is a clinical registry that documents treatments and outcomes for patients with rectal cancer [Citation5]. All patients diagnosed with rectal cancer are reported by the clinician with clinical data regarding diagnosis, preoperative work-up and treatment, and subsequent locoregional recurrences or metastases. The registry is an integrated part of the CRN. In 2007, it was expanded to include all patients diagnosed with colon cancer, and the name was changed to the NCCR. The board of the NCCR comprises the Norwegian Gastrointestinal Cancer Group-Colorectal subgroup (NGICG-CR), which maintains academic responsibility for the registry and the national treatment guidelines for colorectal cancer. The NGICG-CR includes colorectal surgeons, oncologists, radiologist, pathologist, epidemiologist, and representatives from the CRN, also ensuring national geographic representation.
Norwegian treatment guidelines
Since the introduction of TME surgery, rectal cancer treatment in Norway has gradually evolved. Initially, the main focus was on optimising surgery by implementing the TME technique and routines for pathological work-up of the specimens [Citation2,Citation6]. Thereafter, rectal surgery became reserved for gastrointestinal surgery specialists. Guidelines subsequently recommended preoperative chemoradiotherapy for patients with fixed or T4 tumours, and postoperative chemoradiotherapy to patients with bowel perforation near the primary tumour or pT4 tumours. Clinicians gradually adopted the use of recommended preoperative imaging with MRI and treatment discussions in multidisciplinary teams. Since 2003, neoadjuvant chemoradiotherapy was recommended for patients with a tumour with ≤ 3 mm to the mesorectal fascia based on MRI. Staging of the pathologic specimen includes staging after surgery (pTNM) or after chemoradiotherapy and surgery (ypTNM). Adjuvant chemotherapy has not been routinely recommended and is therefore rarely administered. Patients are usually followed for five years after surgery. Screening for colorectal cancer has not been routinely implemented in Norway. The treatment recommendations of the NGICG-CR were officially endorsed by the national health authorities in 2010, with the publishing of comprehensive national guidelines to ensure equal patient treatment regardless of socio-economic status or geographic location.
Patients
The present study included all patients diagnosed with rectal cancer in Norway from 1 November 1993 to 31 December 2010. The data were stratified into five time periods: 1993–1997, 1998–2000, 2001–2003, 2004–2006, and 2007–2010. The time period groupings were selected to avoid periods with too few patients and a corresponding lack of statistical power. During the earliest period, TME was not yet fully implemented in all hospitals, but it has been considered standard treatment in all hospitals since 1998 [Citation5].
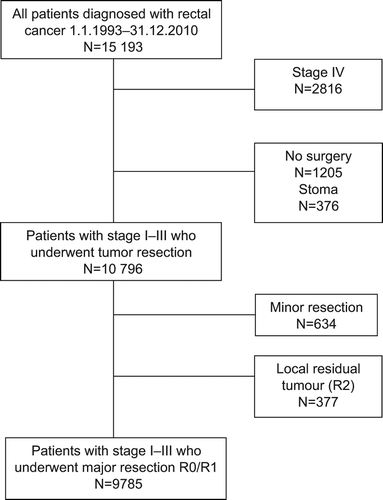
Patients who underwent tumour resection were included in the final analyses (). Metastases were defined as synchronous if detected within four months of diagnosis or surgery. One could anticipate increased sensitivity for detecting metastases during the study period, and thereby a stage migration, however this was not observed. Designated clinicians at each hospital routinely receive queries requesting any missing data regarding preoperative staging, treatment, and updated information on local and distant recurrence for stage I–III patients operated with curative (R0/R1) major resection; these patients were included in the analyses of recurrence rates. For the last time period analysed, clinical information regarding diagnosis and staging was received for 93% of patients, and treatment parameters for 91%. In 2012/2013, a large query to all hospitals was undertaken to ensure updated data on local and distant recurrences. Of 36 hospitals, 31 responded, representing 98% of the patients, who were included in the analyses of subsequent local and distant recurrence.
Statistical methods
To estimate relative survival, patients were followed from inclusion date until death from any cause or the end of the study period (31 December 2012), whichever came first. When analysing all patients diagnosed with rectal cancer the inclusion date was the date of diagnosis, whereas for patients undergoing curative surgery the inclusion date was the date of surgery. To estimate the proportion of patients who experienced local recurrence and/or distant metastasis, patients were followed from the time of surgery until the first recurrence, or until the end of the study period. For groups of patients with at least five years of complete follow-up, the cohort method was used to calculate the estimates. For the last cohort (2007–2010), a variant of the hybrid approach was used to calculate relative survival estimates [Citation14]. This method accounted for the lack of patients with longer follow-up by including previously diagnosed patients who had already survived a given number of years. For the survival analysis, complete follow-up was available for patients diagnosed up to 2010. For local and distant recurrence, complete follow-up was available for patients diagnosed up to 2009.
Time trends were determined using ordinary linear regression. Information on type of surgery was missing for 9.2% of the patients in the last period, due to incomplete clinical reporting. To better be able to study time trends, it was assumed that the distribution of surgery type was equal for those with and without clinical information. A K-sample test for equality of medians was applied to determine trends in ageing. A log-rank test was used to determine differences in survival curves. The significance level was set to 95%, and p-values were calculated where applicable.
Results
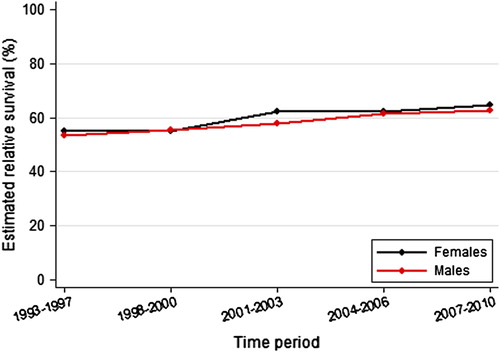
A total of 15 193 patients were diagnosed with rectal cancer from 1993 to 2010; the median age was 72 years, and 57.0% were males. Metastatic disease was present at diagnosis in 2816 patients (18.5%; ), the proportion was similar in all time periods. Of the 12 377 patients with stage I–III disease, 1205 did not undergo surgery, and 376 received a stoma but no tumour resection; these patients had a median age of 80 years. The five-year relative survival for all patients increased from 54.1% in 1993–1997 to 63.4% in 2007–2010 (p < 0.001; ).
A total of 10 796 patients with stage I–III disease underwent tumour resection, and were included in the further analyses (). Patient and treatment characteristics are presented in , stratified for time period. Of these patients, 6219 (57.6%) were male, and the median age was 71 years (69 years in the last time period; p < 0.001).
Table I. Patient and treatment characteristics according to treatment period among all 10 796 patients who underwent tumour resection for rectal cancer from 1993 to 2010.
Low anterior resection (LAR) was performed in 56.2% of cases, abdominoperineal resection (APR) in 26.8%, and Hartmann resection in 7.7% (). Over the time periods, the APR rate decreased slightly with a corresponding increase in the rate of Hartmann resection. The use of defunctioning stomas increased from 27.5% of patients with LAR in 1993–1997 to 50.1% in 2007–2010 (p < 0.001). Minor resection was performed in 634 (5.9%) patients, the median age was 78 years and most had stage I disease. Macroscopic residual primary tumour (R2) was present in 377 (3.5%) patients. Emergency surgery was performed in 1.5% of the cases. During the last time period, 9.5% of patients underwent laparoscopic procedures, with the annual rate increasing from 3.7% in 2007 to 17.1% in 2010 (p < 0.001).
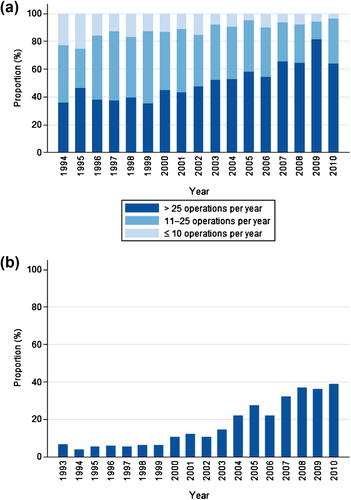
Curative rectal cancer surgery was performed at 56 hospitals in 1993–1997, and at 36 hospitals in 2007–2010. In the last time period, 69% of patients were operated at hospitals that performed > 25 curative operations for rectal cancer per year (). These data show a marked increase in the percentage of patients operated at large-volume hospitals.
Analyses across time periods showed an increasing proportion of patients receiving preoperative radiotherapy or chemoradiotherapy (), from 6.5% of patients in 1993 to 39.0% in 2010. Preoperative radiotherapy was most often administered at a total dose of 50 Gy, with concomitant use of 5- fluorouracil or capecitabine. Postoperative chemoradiotherapy was administered to approximately 5% of patients, and this rate remained stable across the time periods. In the last time period, 2.8% received adjuvant chemotherapy.
Pathological examination of the surgical specimens (i.e. pTNM or ypTNM stage) revealed that 30.8% were stage I, 32.2% stage II, and 29.4% stage III (). In the last time period, routine pathology showed a complete response after chemoradiotherapy in 4.6% of all patients. CRM was reported in 49.7% of patients in the first time period and 81.6% in the last time period. The rate of involved (0–1 mm) CRM decreased from 9.6% to 5.7% across the same periods. The rate of anastomotic leakage after LAR decreased and was 9.2% in the last time period. The 30- and 100-day mortality rates decreased, and were 1.4% and 3.0%, respectively, in the last time period (p < 0.001).
Table II. Disease stage and outcome after surgery according to treatment period among all 10 796 patients who underwent tumour resection for rectal cancer from 1993 to 2010.
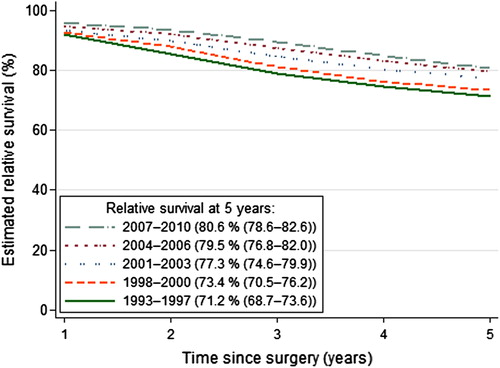
Among the 10 796 patients who underwent tumour resection, the five-year relative survival improved from 71.2% in 1993–1997 to 80.6% in 2007–2010 (p < 0.001; ).
The five-year estimated local recurrence rate in the 9785 patients who had undergone major resection (R0/R1) improved from 14.5% in 1993–1997 to 5.0% in 2007–2009 (p < 0.001; ). The largest improvements in local recurrence rates were seen among patients with p/yp T3 and T4 tumours (). Estimated median time to local recurrence was 20.3 months for all time periods (p = 0.657).
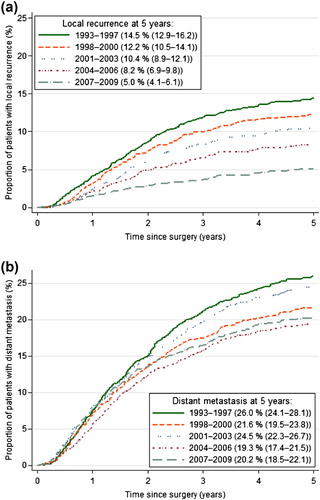
Table III. Local recurrence rates after R0/R1 major resection according to pathological tumour stage and preoperative (chemo)radiotherapy for rectal cancer.
The five-year estimated distant recurrence rate in these patients decreased from 26.0% in 1993–1997 to 20.2% in 2007–2009 (p < 0.001; ). Despite improved local control, the rate of distant metastases remained relatively unchanged the last time periods.
Discussion
The present study demonstrates that the past 20 years of continuous efforts to improve rectal cancer management have significantly improved survival and local and distant recurrence rates on a national level. This has been a joint effort, uniting multidisciplinary professional communities and the CRN, with the common goal of improving outcomes. Important factors contributing to this success were probably the implementation of TME surgery of high quality [Citation2], advancements in high-resolution radiology with better preoperative staging [Citation7], dedicated pathology [Citation6], and increased use of preoperative chemoradiotherapy [Citation12]. The study reports the long-term outcomes from a national population-based rectal cancer registry with a high degree of completeness and a high quality of data. The results from the NCCR document that excellent results were achieved on a national level.
In the early 1990s, rectal cancer treatment underwent a paradigm shift with the introduction of TME surgery and the systematic reporting of detailed pathological data, including CRM [Citation2,Citation6]. Inspired by this, the Norwegian Rectal Cancer Project was initiated with the major objective of reducing local recurrence rates by introducing TME at a national level. In the late 1980s, the reported national local recurrence rates ranged from 21% to 28% [Citation15,Citation16], while lower recurrence rates achieved by TME surgery were reported by Heald et al., and supported by others [Citation2,Citation17,Citation18]. While some countries combined short-course preoperative radiotherapy with surgery [Citation9,Citation10], in Norway, the initial strategy was to improve treatment by optimising surgery. Following the implementation of TME surgery, the first report from the NCCR showed reduction of the local recurrence rate to 13% [Citation5]. Since then, the recurrence rate has steadily decreased, most likely due to further improvements in surgical technique, surgery performed at fewer hospitals, locoregional staging with MRI enabling improved planning of the surgical procedure and selection of patients for neoadjuvant treatment, treatment decisions in multidisciplinary teams, and increased use of preoperative chemoradiotherapy [Citation7,Citation8,Citation12,Citation19,Citation20]. Local recurrence rates of 5% are comparable to results obtained in large randomised studies of primary resectable rectal cancer [Citation9–11], and this achievement on a national population-based level can be considered as very good [Citation21].
The Norwegian Rectal Cancer Registry was the first population-based national clinical registry of rectal cancer. It is based on mandatory reporting of clinical and pathology data, linkage with other population-based registries, and strict routines for prospective quality control including queries regarding missing data and updates on outcomes.These measures ensure a high degree of completeness and quality of core parameters. Several other countries have since established national, regional, or multicentre registries, and a European initiative to provide quality data on rectal cancer in Europe (EURECCA) has been undertaken [Citation22–25]. Well designed and continuously maintained national clinical registries are essential to ensure valid outcome documentation.
Population-based clinical registries have obvious limitations. Despite great efforts, the data are limited to a smaller number of variables, and are of less detailed quality compared to those registered in controlled clinical trials. More sensitive staging methods over time may result in increased detection of metastases and thereby stage migration; however, this was not seen in the present study. Furthermore, the pathological staging reported in the registry is dependant on whether radiotherapy was given or not, and is therefore either pTNM or ypTNM. For registry-based studies, data quality is of the utmost importance. The Swedish Rectal Cancer Registry has demonstrated good validity for several core parameters, few erroneous registrations, and missing data for recurrence in 13% [Citation26], and the NCCR has recently shown good validity [Citation27]. In the present study, in spite of extensive queries, the registered recurrence rates may be underestimated, and may possibly reflect the first occurring event. Furthermore, for the last time period the follow-up time is shorter, and therefore recurrence data are based on observed recurrences and estimates. However, survival data are highly reliable due to the linkage with Statistics Norway based on the unique personal identification number.
Alongside the results of clinical randomised trials and well designed observational studies, population-based data may provide an important contribution as all patients in the defined population are included, regardless of age and possible comorbidities. The data are also very useful for quality improvement, as the NCCR provided each institution with the hospital-specific outcomes alongside national results. Since 2013, the complete annual NCCR report is published on the CRN website, including details regarding the treatment results per individual hospital, in line with national requirements.
Although local control and survival have improved over time [Citation28,Citation29], further improvement in outcome remains a challenge. This could be obtained by earlier cancer detection, increased rate of radical (R0) resection by extended TME, and multimodality treatment of patients with high risk of relapse after initial curative resection, and those who present with metastatic disease. The role of adjuvant chemotherapy in patients with rectal cancer remains controversial, as recent studies of adjuvant chemotherapy after preoperative chemoradiotherapy have not reported improved outcomes [Citation4,Citation30]. Risk factors for poor survival include involved CRM, malignant lymph nodes, T3 tumour with large extramural tumour spread, vascular invasion, and peritoneal affection, often detected by MRI [Citation7,Citation31]. Further studies are needed to address therapeutic responses and effects on survival in patients with high-risk tumour characteristics. One major issue will likely be the potential benefit of neoadjuvant chemotherapy among patients with a high risk of relapse. New prognostic biomarkers might further improve risk stratification and guide treatment decisions.
Early studies from the NCCR documented decreased local recurrence rates following implementation of the TME tecnique [Citation5], and later additional reduction following further optimisation of surgery through specialisation, and increased use of preoperative radiotherapy [Citation20]. Studies from the registry have shown that decreased local and distant recurrence rates have improved the survival of patients with rectal cancer at a national level [Citation28,Citation29]. Poor prognostic factors have been identified, including distance to the CRM, inadvertent perforation, pathological lymph nodes, anastomotic leakage, peritoneal affection, and short distal resection margin [Citation31]. Studies have described the treatment outcomes in young and elderly patients, for T1 and T4 tumours, for recurrent disease treatment, and for palliative treatment [Citation32,Citation33]. The NCCR has provided the basis for population-based research on late effects on quality of life, and anorectal and sexual function [Citation34,Citation35]. These studies on large cohorts from everyday practice may be more representative than patient series from specialised centres, and the published results have been directly used in the development and revisions of national treatment guidelines.
Although many topics have been investigated in the NCCR, research challenges remain. The possibility of increasing the rate of R0 resections by more dedicated surgery should be investigated. Patients with metastatic disease and patients not amenable for curative treatment represent a significant subgroup, often with severe symptoms, and should be further analysed [Citation33]. The treatment of rectal cancer with synchronous resectable metastases has changed dramatically following the introduction of effective combination chemotherapy and extended indications for surgery of resectable metastases in the liver, lung or peritoneum [Citation4]. A proportion of these patients receive multimodality treatment with curative intent, blurring the distinction between curative and palliative treatment, and the outcomes of this treatment should be evaluated. Functional results have not been part of the national registries. It is timely and a national requirement to consider inclusion of patient-reported outcomes.
The present study confirms the considerable improvement of outcomes for patients treated for rectal cancer over the past two decades in Norway. While local control of rectal cancer has improved, the challenge remains to reduce the metastasis rate and thereby further increase survival. Another challenge is tailoring treatment strategies, with the aim of increasing cure rates without impairing functional outcomes. Population-based registries are useful tools for documenting outcomes in all patients with rectal cancer.
Acknowledgements
We thank everyone who has reported data to the NCCR and all previous members of the NCCR board for their great efforts and contributions to improving the outcomes of rectal cancer treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69–90.
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–82.
- Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. Colorectal cancer. Lancet 2010;375: 1030–47.
- Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479–516.
- Wibe A, Moller B, Norstein J, Carlsen E, Wiig JN, Heald RJ, et al. A national strategic change in treatment policy for rectal cancer – implementation of total mesorectal excision as routine treatment in Norway. A national audit. Dis Colon Rectum 2002;45:857–66.
- Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994;344:707–11.
- Taylor FG, Quirke P, Heald RJ, Moran BJ, Blomqvist L, Swift IR, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 2014;32:34–43.
- MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis 2009;11:291–5.
- Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980–7.
- van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–82.
- Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009;373:811–20.
- Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Pahlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687–94.
- Larsen IK, Smastuen M, Johannesen TB, Langmark F, Parkin DM, Bray F, et al. Data quality at the Cancer Registry of Norway: An overview of comparability, completeness, validity and timeliness. Eur J Cancer 2009;45:1218–31.
- Brenner H, Rachet B. Hybrid analysis for up-to-date long-term survival rates in cancer registries with delayed recording of incident cases. Eur J Cancer 2004;40:2494–501.
- Soreide O, Norstein J. Local recurrence after operative treatment of rectal carcinoma: A strategy for change. J Am Coll Surg 1997;184:84–92.
- Dahl O, Horn A, Morild I, Halvorsen JF, Odland G, Reinertsen S, et al. Low-dose preoperative radiation postpones recurrences in operable rectal cancer. Results of a randomized multicenter trial in western Norway. Cancer 1990;66:2286–94.
- Bjerkeset T, Edna TH. Rectal cancer: The influence of type of operation on local recurrence and survival. Eur J Surg 1996;162:643–8.
- Lange MM, Martz JE, Ramdeen B, Brooks V, Boachie-Adjei K, van de Velde CJ, et al. Long-term results of rectal cancer surgery with a systematical operative approach. Ann Surg Oncol 2013;20:1806–15.
- Wibe A, Eriksen MT, Syse A, Tretli S, Myrvold HE, Soreide O. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg 2005;92:217–24.
- Hansen MH, Kjaeve J, Revhaug A, Eriksen MT, Wibe A, Vonen B. Impact of radiotherapy on local recurrence of rectal cancer in Norway. Br J Surg 2007;94:113–8.
- Tiefenthal M, Nilsson PJ, Johansson R, Glimelius B, Pahlman L. The effects of short-course preoperative irradiation on local recurrence rate and survival in rectal cancer: A population-based nationwide study. Dis Colon Rectum 2011;54:672–80.
- Pahlman L, Bohe M, Cedermark B, Dahlberg M, Lindmark G, Sjodahl R, et al. The Swedish rectal cancer registry. Br J Surg 2007;94:1285–92.
- Bulow S, Harling H, Iversen LH, Ladelund S. Improved survival after rectal cancer in Denmark. Colorectal Dis 2010;12:e37–42.
- Breugom AJ, Boelens PG, van den Broek CB, Cervantes A, van Cutsem E, Schmoll HJ, et al. Quality assurance in the treatment of colorectal cancer: The EURECCA initiative. Ann Oncol 2014;25:1485–92.
- van den Broek CB, van Gijn W, Bastiaannet E, Moller B, Johansson R, Elferink MA, et al. Differences in pre-operative treatment for rectal cancer between Norway, Sweden, Denmark, Belgium and the Netherlands. Eur J Surg Oncol 2014;40:1789–96.
- Jorgren F, Johansson R, Damber L, Lindmark G. Validity of the Swedish Rectal Cancer Registry for patients treated with major abdominal surgery between 1995 and 1997. Acta Oncol 2013;52:1707–14.
- Sakkestad ST, Olsen BC, Karliczek A, Dahl O, Pfeffer F. Quality of Rectal Cancer Registry data at a major Norwegian hospital 1997–2005. Acta Oncol 2015 (forthcoming).
- Bernstein TE, Endreseth BH, Romundstad P, Wibe A. Improved local control of rectal cancer reduces distant metastases. Colorectal Dis 2012;14:e668–78.
- Nedrebo BS, Soreide K, Eriksen MT, Dorum LM, Kvaloy JT, Soreide JA, et al. Survival effect of implementing national treatment strategies for curatively resected colonic and rectal cancer. Br J Surg 2011;98:716–23.
- Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: Long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184–90.
- Eriksen MT, Wibe A, Haffner J, Wiig JN. Prognostic groups in 1,676 patients with T3 rectal cancer treated without preoperative radiotherapy. Dis Colon Rectum 2007;50:156–67.
- Endreseth BH, Romundstad P, Myrvold HE, Hestvik UE, Bjerkeset T, Wibe A. Rectal cancer in the young patient. Dis Colon Rectum 2006;49:993–1001.
- Sigurdsson HK, Korner H, Dahl O, Skarstein A, Soreide JA. Palliative surgery for rectal cancer in a national cohort. Colorectal Dis 2008;10:336–43.
- Guren MG, Eriksen MT, Wiig JN, Carlsen E, Nesbakken A, Sigurdsson HK, et al. Quality of life and functional outcome following anterior or abdominoperineal resection for rectal cancer. Eur J Surg Oncol 2005;31:735–42.
- Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, et al. Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:1005–11.