Abstract
Background. Patients with advanced stage lung cancer and somatic mutations in the epithelial growth factor receptor (EGFR) gene are currently treated with tyrosine-kinase inhibitors. The Norwegian Lung Cancer Group (NLCG) recommended EGFR testing of all patients with non-small cell lung carcinoma (NSCLC) from June 2010. From March 2013, testing of squamous cell carcinomas was terminated. We have analysed how these recommendation were followed at a medium-sized Norwegian hospital and we present data on mutation frequency, retesting and possible explanations for missing test results.
Material and methods. All pathology reports for patients diagnosed with NSCLC at Vestfold Hospital Trust were examined for the period June 2010 to December 2013. Mutation analyses were done at the Department of Pathology, Oslo University Hospital.
Results. Material was sent for EGFR analysis for 256 of the 304 eligible patients diagnosed in the period. Material from 48 patients was never sent for EGFR testing, of which five samples consisted of too few tumour cells. For the rest, no obvious reason for omitting EGFR mutation analyses was identified. During the first six months of our study period, material from 25 of 66 NSCLC patients (38%) was not tested, whereas only six of the 118 patients (5%) in 2013 were not tested. For 34 patients, the first tissue specimen contained too few tumour cells and a new sample was sent for EGFR analyses for 11 of these. EGFR mutation was detected in 7.1% of the analysed NSCLC and in 9.4% of adenocarcinomas.
Discussion. Especially for patients with advanced stages of NSCLC, EGFR mutation status is necessary for treatment stratification. Our results show that the guidelines were followed increasingly over time for patients diagnosed with NSCLC at the Vestfold Hospital Trust. The establishment of interdisciplinary meetings has improved the diagnostic routines.
In 2013, 2856 patients were diagnosed with lung cancer in Norway and 2162 died the same year of the disease [Citation1]. Lung cancer represented approximately 10% of all new cancer cases and 20% of all cancer deaths. The disease accounts for approximately 5% of all deaths in Norway; and in 2012 lung cancer took about as many years of life (almost 33 000 years of life) as breast, prostate and colon cancer combined (just over 34 000 years of life) [Citation1,Citation2]. An aggressive biology is the main reason for the dismal prognosis with a five-year survival rate for men of 13% and 19% for women [Citation1]. Another reason is that approximately 70% of the patients are diagnosed with metastatic disease where curative treatment is not available [Citation3].
Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers with adenocarcinoma, squamous cell carcinoma and large cell lung cancer as the most common subtypes. Until recently, the only relevant treatment for the majority of NSCLC patients with metastatic disease has been palliative radiotherapy and/or chemotherapy. Median survival with such treatment is shown to be around seven months in Norwegian Phase III studies with over 1300 patients enrolled [Citation4,Citation5].
Over the last years, several studies clearly concluded that the presence of specific mutations in the epithelial growth factor receptor (EGFR) gene is associated with good effect of the tyrosine-kinase inhibitors erlotinib, gefitinib or afatinib [Citation6–9]. Patients with EGFR mutation positive lung cancer treated with tyrosine-kinase inhibitors have a survival rate of about two years [Citation10,Citation11]. Patients without such mutations have little if any advantage of these drugs [Citation12].
In addition to the EGFR mutations, several other molecular aberrations are of clinical importance in lung cancer, such as ALK translocations [Citation13].
Personalised treatment, based on identification of specific molecular aberrations, including EGFR mutations, are standard for advanced NSCLC today. However, diagnostics are done by various methods (FISH, immunohistochemistry, DNA analysis) which entail increased requirements for quality and quantity of tumour material. This can be challenging for small biopsies or sparse cytological samples from bronchoscopy, pleural fluid examination or image-guided biopsies [Citation14,Citation15]. Most tissue samples available for diagnostics of lung carcinomas are taken by bronchoscopy (biopsy, brush, bronchoalveolar lavage and fine needle aspirate cytology) with or without endobronchial ultrasound (EBUS). In addition image-guided core biopsies are used.
Only in 2013 came the first international guideline for the treatment and analysis of small tissue samples, as well as interpretation of the test results [Citation16]. From 2011, Oslo University Hospital conducted EGFR mutation analyses on cytological samples when biopsy material was missing.
The national expert group for lung cancer, Norwegian lung cancer group (NLCG) (www.nlcg.no), recommended routine testing for mutations in the EGFR gene of all non-small cell lung carcinomas from 2010 [Citation17]. A later evaluation revealed that only 0.5% of the squamous cell carcinomas were EGFR mutated and therefore routine testing for this group was terminated from March 2013. Routine testing for ALK-aberrations of non-squamous NSCLC was established in 2014.
In this retrospective study we wish to examine if patients were tested for EGFR mutation and possible causes for missing results.
Material and methods
In excess of 240 000 people are covered by Vestfold Hospital Trust, Tønsberg. Patients diagnosed with NSCLC at Vestfold Hospital Trust during the period June 2010 to December 2013 were identified using the pathology system DocuLive Pathology. Clinical information (histology, stage, treatment and course of disease) and results of mutation analyses done at Oslo University Hospital were registered for all patients. The project is approved by the Data Protection Authority as a Quality Assurance Project (project number 38913).
Tumour specimens are sent from Vestfold Hospital Trust for EGFR testing at Unit of Molecular Pathology, Department of Pathology, Oslo University Hospital. The mutation analysis was performed by real-time PCR using a commercial kit. Before January 2013 this was done with TheraScreen (Qiagen), which analyses 28 of the most commonly occurring genetic changes. Oslo University Hospital had a requirement of at least 20% tumour cells in the sample until January 2013, while they used TheraScreen (Qiagen). From 2013, the Cobas method (Roche) was used, which analyses 41 changes in the same exons. This kit requires a minimum tumour cell ratio of 5–20%, and samples with tumour cell content above 10% have since then been analysed.
For samples without EGFR mutation results, we registered possible explanations including the patient's disease stage and general condition, procedure for sampling material, level of experience of the doctor who performed the procedure and if repeated biopsy attempts were made. We also wanted to compare if the proportion of rejected samples was linked to the method of sampling (cytology or biopsy).
Results
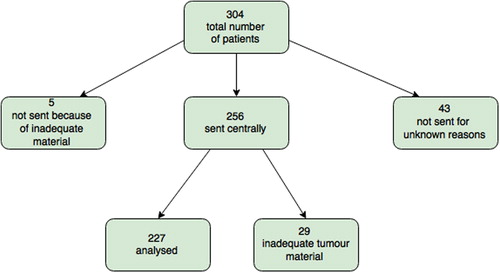
We recorded 304 cases of NSCLC from June 2010 to December 2013. The first two years, cytological samples were not sent for testing due to uncertainty about cytological smears for mutation testing. In recent years cytological samples have proven to be suitable for EGFR mutation testing [Citation18–22], and the pathologist now routinely sends both biopsies and cytological samples for mutational analyses.
Of the 304 patient samples, five were not sent for EGFR testing due to inadequate tumour material and 43 were not submitted without any specific reasons given (). Of these 43 samples, 25 were among the 66 patients diagnosed with NSCLC during the first six months of our study period (38%), whereas only six samples were from the 118 patients with non-squamous NSCLC diagnosed in 2013 (5%).
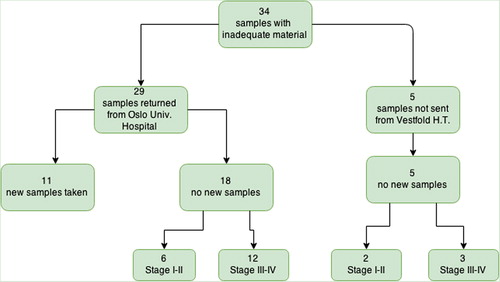
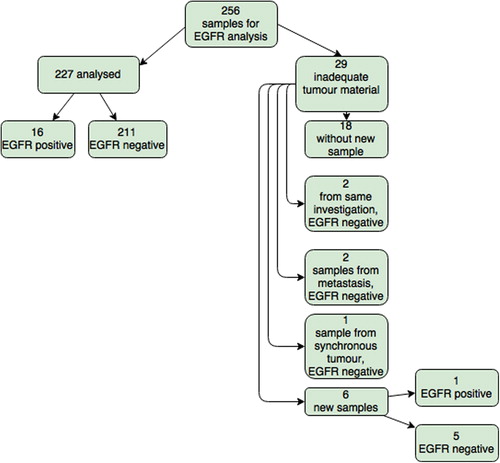
For a total of 34 patients (13%) the first biopsy were not analysed at department of molecular pathology due to inadequate tumour material (determined locally for five patients, and centrally for 29 patients). For 11 of the 34, a new samples was collected and sent for EGFR analysis (35%) (). A detailed overview of the 11 re-submitted samples can be found in . One of the 11 was diagnosed with activating EGFR mutation in the second sample submitted.
EGFR testing was conducted in a total of 238 (256 submitted, 29 returned and 11 re-submitted) patients and mutation was detected in 17 cases (7.1%). There were 16 (9.4%) mutation positive in the 170 patients with adenocarcinoma. The mutations found were one exon 18 (G719X), eight exon 19 deletions, six exon 21 L858R, one exon 21 L861Q and one unknown. One (2%) of the 49 patients with squamous cell carcinoma was found to have an EGFR mutation. No change in frequency was seen when change of method from TheraScreen to Cobas, however the numbers are small.
Test results from the EGFR analyses were received after 17 days in median (range 8–36 days) from sampling. In our material, the samples were sent to Oslo University Hospital after a median of 6.5 days (range 4–8) from sampling, as material was not sent before a histological diagnosis was established. When the material was unsuitable for molecular analyses, a new sample was obtained, submitted and the test result received after a median of 38 days (range 17–69).
In total 11 of the 23 patients with missing EGFR result (rejected and not re-submitted) were in disease stage I and II and received curative treatment. Ten underwent surgical treatment and one had stereotactic radiosurgery. Additional samples were not collected by Vestfold Hospital Trust due to lack of therapeutic consequences. Six of the 10 surgically treated patients had an EGFR test performed on the surgical specimen, of which none were mutated.
Of the four patients in stage IIIA without EGFR result, two were re-biopsied with negative results. Of the five patients who were not sent from Vestfold Hospital Trust due to inadequate tumour material, three patients were in stage I–III and two in stage IV. New tissue samples were not collected.
A total of 13 of the 15 stage IV patients without EGFR mutation analysis (rejected or not sent) were not re-biopsied. Three of these patients did not want active treatment and one was not a candidate for active treatment because of very poor general condition. One patient was not re-biopsied due to rapid disease progression. Eight patients were never considered for re-biopsies, including a young patient who had brain metastases at the time of diagnosis, but who lived for 19 months after diagnosis.
Altogether, 230 biopsy samples and 26 cytology specimens were submitted (all 26 are EBU-guided transbronchial needle aspiration). Only one cytology sample contained too few tumour cells for EGFR mutation analysis.
Experienced physicians usually sampled enough tumour cells, with only 2.8–8% returned samples due to low tumour tissue content. The less experienced had 9–50% samples with too few tumour cells for EGFR analysis ().
Table I. Percentage of returned samples in relation to experience of the physician.
For image supervised biopsies, non-analysable samples were more frequent when puncturing small (< 3 cm) peripheral tumours than when taken from large central tumours.
Of 14 image-guided biopsies that were returned because of inadequate tumour tissue only two were procedures with complications: one with bleeding and the other with pneumothorax. Both tumours were peripheral. Of three bronchoscopic biopsies that were returned due to inadequate tumour tissue, one was complicated by major bleeding; in another the patient was very restless during the procedure. The last procedure was without complications.
Since February 2014, all patients with lung cancer have been discussed in interdisciplinary meetings at Vestfold Hospital Trust with a pulmonologist, pathologist, radiologist, oncologist, and patient coordinator present. Pulmonologist is at once notified by pathologist if the specimen is inadequate for EGFR analysis. In addition, NSCLC patients with an inadequate first biopsy are discussed.
Discussion
This study presents the results of a retrospective review of the first three and half years of routine analyses of EGFR mutations in patients with NSCLC at Vestfold Hospital Trust. Our aim was to analyse how the new guidelines of EGFR testing of NSCLC were implemented at a medium-sized Norwegian hospital.
We have used medical records from Vestfold Hospital Trust to identify relevant patients. In excess of 240 000 people are covered by Vestfold Hospital Trust in the study time period. Statistics from Cancer Registry of Norway shows that in Vestfold 153 lung cancer cases were diagnosed (all histologies, small cell and non-small cell) in 2010, 182 in 2011, 163 in 2012 and 175 in 2013 [Citation1]. This corresponds well with our figures for patients eligible for EGFR testing at Vestfold Hospital Trust.
Adenocarcinomas were EGFR mutated in 9.4%. This is consistent with publications from Norway and other European countries. The incidence of EGFR mutation in adenocarcinomas for Norway is 11% [Citation23] and 10.1% [Citation24]. Published figures for France are 9.5% [Citation25] and Denmark 8% [Citation22].
Fifteen samples from patients with metastatic NSCLC in our material were returned without EGFR mutation results. Of these, only two had a new biopsy taken. In addition, a proportion was never submitted for analysis, albeit fewer in recent times. It is important to strive for EGFR results on everyone as approximately 10% of patients with NSCLC have an activating EGFR mutation and thus could benefit from treatment with tyrosine kinase inhibitors.
From 256 submitted samples, 29 (13%) were rejected from further analyses due to low number of tumour cells. This is in accordance with other studies, as Pang et al. [Citation26] reported 11.9% rejected histological samples and 10.9% rejected cytological samples, while Twaddell [Citation27] reported 13% returned tests because of the inadequate tumour material. Some centres have up to 28% (18/64) samples without sufficient tumour content to perform analysis [Citation28]. A Danish report, however, reported 6.5% samples with poor quality or insufficient tumour material, possibly because they analyse samples without having a minimum requirement for the percentage tumour cells in the sample [Citation22].
There are differences between laboratories regarding pre-analytical requirements for material quality. This concerns both absolute amount of tumour cells and the percentage of tumour cells compared to normal cells in the specimen, and the requirements are influenced by analytical method. In the beginning sequencing was the “gold standard”, a technique that involves screening for all possible mutations in the studied area of the gene, but where the sensitivity is limited so that tumour cell percentage should be at least 25%. University hospitals in Norway now use commercial kits that analyse only known mutations, and samples with tumour cell content above 10% are analysed. The other regional hospitals also analyses samples with tumour cell percentage between 10% and 20%, sometimes even below 10%, but with the reservation that negative result does not preclude EGFR mutation. At Haukeland University Hospital, 12% of the samples had < 20% tumour cell percentage in the years 2012–2013 (pers. comm. from pathologist Lars Helgeland). There was no difference in mutation frequency in samples containing fewer or more than 20% tumour cells. This is consistent with other published results, which indicates that the quality of the material (fixation and preparation methods for DNA) is as important as proportion of tumour cells or absolute cell numbers and is the limiting factor to achieve [Citation29,Citation30]. Identification of EGFR mutation is important for physicians in order to treat patients accordingly. Erlotinib is approved as second-line treatment when unknown EGFR status, but in practice patients with EGFR wild-type are rarely treated with erlotinib at our hospital.
Since February 2014, all patients with lung cancer are discussed in interdisciplinary meetings at Vestfold Hospital Trust. Pulmonologist is at once notified by pathologist if the first tumour specimen is inadequate for EGFR analysis. In these cases we jointly discuss the need and possibility for new sampling.
Bronchoscopic biopsies gave better results than image-guided lung biopsies in our material. This was also reported by Subramonia Iyer et al. [Citation28].
For small peripheral tumours, the frequency of non-analysable samples was higher than for large central tumours taken by image-guided biopsy. It is known that small tumours in motion can be challenging to hit.
The quality of image-guided biopsies taken by more experienced physicians is better than those taken by doctors with less experience. The complication rate also decreases with increasing experience. Therefore, it is important to have good training and guidance of interventional physicians.
Cytological material and biopsies are now considered equally suitable for molecular analysis [Citation18,Citation19,Citation21,Citation22,Citation31] making individualised treatment options possible for patients with NSCLC where tumour biopsies are not available. Cytological samples accounts for a third of all samples tested in Japan [Citation19].
For patients with rejected samples, EGFR results were available after 17–69 days (median 38) from rejection of the first sampling. For this patient group, with poor prognosis and often rapid disease progression, one should strive for a quicker turn- around-time. It is desirable with faster feedback from molecular pathological laboratory if the sample is unsuitable, so the patient can have a new sampling as quick as possible. Interdisciplinary meetings can improve logistics [Citation32].
In conclusion, we have shown that the EGFR testing routines for NSCLC generally are followed according to the national guidelines at a medium-sized Norwegian hospital like Vestfold Hospital Trust. It takes time to adjust to new routines, and interdisciplinary communication is of utmost importance when guidelines change.
Acknowledgements
This work was funded in part from grants from Helse Sør-Øst and “The Norwegian Cancer Society's national milieu of expertise for lung cancer” This article uses data from the Cancer Registry of Norway. Interpretation and reporting of these data are the authors’ responsibility alone and have not been subject to approval from the Cancer Registry of Norway.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cancer in Norway 2012. Cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer Registry of Norway; 2014. p. 97.
- Brustugun OT, Moller B, Helland A. Years of life lost as a measure of cancer burden on a national level. Br J Cancer 2014;111:1014–20.
- Sagerup CMT, Smastuen M, Johannesen TB, Helland A, Brustugun OT. Sex-specific trends in lung cancer incidence and survival: A population study of 40,118 cases. Thorax 2011;66:301–7.
- Helbekkmo N, Sundstrom SH, Aasebo U, Brunsvig PF, von Plessen C, Hjelde HH, et al. Vinorelbine/carboplatin vs gemcitabine/carboplatin in advanced NSCLC shows similar efficacy, but different impact of toxicity. Br J Cancer 2007;97:283–9.
- Grønberg BH, Bremnes RM, Fløtten Ø, Amundsen T, Brunsvig PF, Hjelde HH, et al. Phase III study by the Norwegian lung cancer study group: Pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:3217–24.
- Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. New Engl J Med 2009;361:947–57.
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. New Engl J Med 2010;362: 2380–8.
- Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–34.
- Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239–46.
- Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. New Engl J Med 2009;361:958–67.
- Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong S-S, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–74.
- Garassino MC, Martelli O, Broggini M, Farina G, Veronese S, Rulli E, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): A randomised controlled trial. Lancet Oncol 2013;14:981–8.
- Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol 2013;31:1097–104.
- Herth FJF, Bubendorf L, Gutz S, Morresi-Hauf A, Hummel M, Junker K, et al. [Diagnostic and predictive analyses of cytological specimens of non-small cell lung cancer: strategies and challenges]. Pneumologie 2013;67:198–204.
- Warth A, Bubendorf L, Gutz S, Morresi-Hauf A, Hummel M, Junker K, et al.[Molecular pathological diagnosis in cytopathology of non-small-cell lung cancer. Standardization of specimen processing]. Pathologe 2013;34:310–7.
- Lindeman NI, Cagle PT, Beasley MB, Chitale DA, Dacic S, Giaccone G, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: Guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol 2013;8:823–59.
- Brustugun O, Helland Å, Fjellbirkeland L, Kleinberg L, Ariansen S, Jebsen P, et al. [Mutation testing for non-small-cell lung cancer]. Tidsskr Nor Laegeforen 2012;132:952–5.
- Ellison G, Zhu G, Moulis A, Dearden S, Speake G, McCormack R. EGFR mutation testing in lung cancer: A review of available methods and their use for analysis of tumour tissue and cytology samples. J Clin Pathol 2013;66:79–89.
- Hagiwara K, Kobayashi K. Importance of the cytological samples for the epidermal growth factor receptor gene mutation test for non-small cell lung cancer. Cancer Sci 2013;104:291–7.
- Jurado J, Saqi A, Maxfield R, Newmark A, Lavelle M, Bacchetta M, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196–202.
- Khode R, Larsen DA, Culbreath BC, Parrish S, Walker KL, Sayage-Rabie L, et al. Comparative study of epidermal growth factor receptor mutation analysis on cytology smears and surgical pathology specimens from primary and metastatic lung carcinomas. Cancer Cytopathol 2013;121:361–9.
- Skov BG, Hogdall E, Clementsen P, Krasnik M, Larsen KR, Sorensen JB, et al. The prevalence of EGFR mutations in non-small cell lung cancer in an unselected Caucasian population. Acta Pathol Microbiol Immunol Scand 2015;123:108–15.
- Helland Å, Skaug HM, Kleinberg L, Iversen ML, Rud AK, Fleischer T, et al. EGFR gene alterations in a Norwegian cohort of lung cancer patients selected for surgery. J Thorac Oncol 2011;6:947–50.
- Kleinberg L, Jebsen P, Lund-Iversen M, Helland A, Fjellbirkeland L. EGFR-mutated lunge cancer in a general Norwegian population. In: (ERS) ERS, editor. European Respiratory Society (ERS) International Congress 6–10 September 2014 Germany, Bayern, Munich.
- Barlesi F, Blons H, Beau-Faller M, Rouquette I, Ouafik L, Mosser J, et al. Biomarkers (BM) France: Results of routine EGFR, HER2, KRAS, BRAF, PI3KCA mutations detection and EML4-ALK gene fusion assessment on the first 10,000 non-small cell lung cancer (NSCLC) patients (pts). J Clin Oncol 2013;1.
- Pang B, Dettmer M, Ong CW, Dhewar AN, Gupta S, Lim GL, et al. The positive impact of cytological specimens for EGFR mutation testing in non-small cell lung cancer: A single South East Asian laboratory's analysis of 670 cases. [Erratum appears in Cytopathology 2012;23:422. Note: Matthias, D (corrected to Dettmer, M)]. Cytopathology 2012;23:229–36.
- Twaddell S, Cox Y. A single centre review of endothelial growth factor receptor (EGFR) mutation testing: A regional Australian experience. J Thorac Oncol 2013;8:S1025–6.
- Subramonia Iyer S, Olsen B, Hrinczenko B. Biopsy method and sample adequacy for molecular testing in advanced non small cell lung cancer. J Thorac Oncol 2012;4:S307–8.
- Krawczyk P, Ramlau R, Chorostowska-Wynimko J, Powrozek T, Lewandowska MA, Limon J, et al. The efficacy of EGFR gene mutation testing in various samples from non-small cell lung cancer patients: A multicenter retrospective study. J Cancer Res Clin Oncol 2015;141:61–8.
- Patton S, Normanno N, Blackhall F, Murray S, Kerr KM, Dietel M, et al. Assessing standardization of molecular testing for non-small-cell lung cancer: Results of a worldwide external quality assessment (EQA) scheme for EGFR mutation testing. Br J Cancer 2014;111:413–20.
- Jurado J, Saqi A, Maxfield R, Newmark A, Lavelle M, Bacchetta M, et al. The efficacy of EBUS-guided transbronchial needle aspiration for molecular testing in lung adenocarcinoma. Ann Thorac Surg 2013;96:1196–202.
- Murgu S, Colt H. Role of the pulmonologist in ordering post-procedure molecular markers in non-small-cell lung cancer: Implications for personalized medicine. Clin Lung Cancer 2013;14:609–26.