Abstract
Background: Neoplasm seeding is a serious complication after liver metastases biopsy. Reported incidences vary between 10% and 19% for colorectal cancer (CRC) and are unknown for breast cancer (BC). The aim of this retrospective study was to determine the frequency of tumor seeding after ultrasound-guided percutaneous biopsy of CRC and BC liver metastases.
Material and methods: Unselected liver biopsies performed in the period of 2005–2012 at our institution were extracted from the National Pathology Registry. Medical records including imaging from patients with biopsy-verified BC and CRC liver metastases were retrospectively reviewed. The endpoint was the development of abdominal wall recurrence following liver biopsy.
Results: Of total 2981 biopsies we identified 278 patients with CRC and 155 patients with BC biopsy-verified liver metastases. During the median follow-up of 25 months after biopsy (range 3–253 months), no seeding was recorded in patients with BC. Within the median follow-up of 34 months (3–111 months), seeding was registered in 17/278 (6%) of patients with CRC; three patients of 278 (1%) had undoubtedly biopsy-related seeding, which became apparent six, nine, and 26 months after biopsy, respectively; and in nine patients (3%) seeding occurred due to either biopsy or other interventions; and five patients had seeding, which were assessed as a consequence of other invasive procedures than biopsies. The median overall survival of the 17 patients with seeding was 70 months compared to 39 months of patients without seeding.
Conclusions: The results showed no seeding in BC patients. Seeding rate after biopsy in CRC patients is not negligible, however, without affecting outcome.
In the era of explosive development of personalized targeted medicine acquisition of tumor tissue by biopsy is necessary in order to optimize efficient treatment decisions. Different studies suggest that primary tumor and their metastases can have different genomic profile, which nourishes interest for metastases biopsy in patients with colorectal cancer (CRC) and breast cancer (BC) [Citation1,Citation2]. One of the potential serious complications after percutaneous biopsy is tumor seeding which is poorly understood and documented. Neoplasm seeding is defined as a local implantation of tumor cells by contamination of instruments and surgical equipment resulting in local growth of the cells and tumor formation (according to the National Library of Medicine of the National Institutes of Health, USA). Frequency and mechanisms behind tumor seeding after biopsy are poorly elucidated; and reports regarding seeding after liver biopsy in patients with BC are not available. In the four largest surveys the range of the needle tract seeding after biopsy of abdominal lesions was miniscule (0–0.009%) [Citation3–6]. Common to these studies was that they were based on patient and doctor reporting, without performing an active cross-sectional imaging verified tracing of seeding; and therefore true seeding rate was probably greatly underestimated. Sparsely documented incidences in the more recent studies of the CRC neoplasm seeding following liver biopsy vary between 10% and 19% [Citation7–9]. These data consist primarily of case series and reports limited by small sample size. Thus, it is still under discussion if seeding is a real problem of concern. Some authors suggest that invasive diagnostic procedures should be carefully considered due to risk of dissemination of cancer cells which can change a potentially resectable localized cancer to an unresectable one [Citation10,Citation11]. Neoplasm seeding has been a subject of particular concern for liver surgeons as a growing number of reports imply a high risk of iatrogenic local spread of cancer after biopsies. Experimental studies have shown cancer cell leakage in the needle tract in the majority of biopsy cases (65–90%) [Citation12,Citation13]. Furthermore, examination of biopsy needles in experimental studies has demonstrated a huge rate of malignant contamination. Tumor seeding has also been reported after radiofrequency ablative procedures [Citation14]. In contrast to experimental findings, implantation metastases in humans seem to be a relatively rare complication, with fluctuating reported incidence depending on the nature of tumor cells, microenvironment, and individual immune response [Citation15].
The purpose of this study was to determine the frequency of tumor seeding after ultrasound (US)-guided percutaneous biopsy of BC and CRC liver metastases and identify seeding consequences with regard to effect on outcome.
Material and methods
Data source and ethics
The Danish National Pathology Register (http://www.patobank.dk/) provides information about all pathology diagnoses since 1999 in Denmark. Data extraction from Patobank restricted to the period of 2005–2012 resulted in 2981 US-guided percutaneous liver biopsies, carried out at our institution. These biopsies were reviewed to identify patients who had verified CRC and BC liver metastases. Retrospective analysis of electronic medical records of these patients was performed. Data quality and reliability were validated by independent reviewers (IC, TL, CN, BS and DN). The protocol was approved by the Institutional Ethics Committee.
Patient population
In total, 2981 liver biopsies were reviewed and patients fulfilling the following criteria were identified: 1) patients with BC or CRC; 2) biopsy-verified BC or CRC liver metastases; 3) post-biopsy follow-up duration ≥3 months, 4) presence of cross-sectional images [computed tomography (CT), positron emission tomography (PET)-CT or magnetic resonance imaging (MRI)] during follow-up. Electronic medical records and all available imaging modalities (CT, PET-CT and MR), performed as a part of surveillance program or treatment control, were reviewed. The endpoint was the development of abdominal wall recurrence after liver biopsy, defined as a new tumor formation either in the cutaneous/subcutaneous soft tissue, muscle or involved peritoneum, pleura or costae within a maximum distance of 10 cm from the site of biopsy path. After thorough review and intrinsic independent interobserver verification all suspected cases were distributed into three categories; 1) certainly derived from the biopsy; 2) other causes (surgery, drainage, radiofrequency ablation) could also be an option; 3) unlikely resulting from the biopsy because of the distance between cancer nodules and the biopsy path was more than 10 cm.
New liver neoplastic lesions along the biopsy needle tract were not considered as a positive seeding since it was hard to differentiate whether it was due to seeding or progression. For the same reason new peritoneal carcinosis (both local peritoneal plaques and widespread) was registered as a post-biopsy event and not seeding.
Methods
All biopsies were performed by experienced interventional radiologists under US guidance using either a 1.2 mm biopsy system for histology [core needle biopsy (CNB)] or a simple 0.8 mm needle for cytology [fine needle aspiration biopsy (FNAC)]. Number of biopsies (needle passes) per procedure was 1–3 depending on the macroscopic outcome of each needle pass. Most of included patients had been exposed to several biopsies in the course of the disease. No specific biopsy (i.e. co-axial procedure) technique was performed to eliminate or reduce seeding. Biopsy outcome was registered in one of these categories: benign changes, no representative material, suspicion of malignancy and carcinoma.
Statistical analysis
Study variables were described using standard summary statistics. All variables were reported as the median (range). All statistical analyses were performed using the SPSS 20.0 for windows statistical package.
Results
Patient characteristics
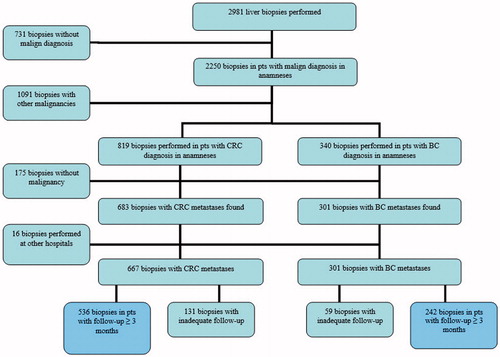
Between 2005 and 2012, of a total 2981 liver biopsies were registered in Patobank. Schematic representation of the working process is presented in . Of these biopsies, 731 procedures performed in patients with no malignant diagnosis and 1091 biopsies with other malignancies than CRC or BC were excluded. Furthermore, 175 biopsies in patients with verified primary CRC or BC were excluded due to absence of malign changes in the biopsied liver material during the course of disease. Sixteen biopsies performed at other hospitals were also excluded. In addition, 131 CRC and 59 BC biopsies in patients with short follow-up of <3 months were not included in the analysis. As a result, 536 and 242 liver biopsies were found, with verified CRC and BC liver metastases, respectively. These liver biopsies represented 278 CRC and 155 BC eligible patients with at least one positive malignant liver sampling. Biopsies of these patients performed before 2005 were additionally included in final analysis.
Breast cancer group
A total of 155 patients were registered with BC. Patient characteristics are displayed in . The median age at diagnosis was 51 years (range 25–88 years). Time from primary diagnosis to first metastatic recurrence was 39 months (range 0–228 months). At a median follow-up of 25 months (range 3–253 months), 55 (35.5%) patients were still alive, 100 (64.5%) were dead, and nobody was lost to follow-up.
Table I. Breast cancer patients and disease characteristics.
Colorectal cancer group
At data cut-off, 278 patients were analyzed. Patient and disease characteristics as well as treatment modalities are listed in . The median age at primary diagnosis of patients with CRC was 64 years (range 35–92 years). The colon and rectum was involved in 201 (72.3%) and 76 (27.3%) patients, respectively. In one case primary tumor localization was not specified. At the time of initial diagnosis 207 (74.5%) patients had metastatic disease. Average time to the first recurrence was 5.5 months (range 0–137 months). Almost all patients (94.6%) had been exposed to chemotherapy, with average number of regimens inclusive re-challenge of 3 (range 0–9). Of all analyzed patients, 103 (37.1%) patients underwent surgical liver resection and 126 (45.3%) patients had undergone radiofrequency ablation. At a median follow-up of 34 months (range 3–111 months), 75 (27%) patients were alive, 203 (73%) were dead, and none were lost to follow-up.
Table II. Colorectal cancer patients and disease characteristics.
Biopsies, associated seeding and outcome
Biopsies and outcomes are displayed in . In the BC group a total of 283 biopsy attempts (mean 1.8 biopsies per patient) were identified. After biopsy 19 (12.3%) and 39 (25.2%) patients with BC were diagnosed with carcinosis and ascites, respectively. After scrupulous review no seeding was registered in patients with BC.
Table III. Biopsies, associated seeding and outcome.
A total of 672 biopsy attempts (mean 2.4 attempts per patient) were registered in CRC group. All cases with post-biopsy CRC seeding are shown in Supplementary Table I (available online at http://www.informahealthcare.com). Totally, 17 of 278 patients (6%) from the CRC group developed seeding suspicious findings on imaging. All cases except one were histologically verified. Three patients (1%), according to our criteria, had undoubtedly biopsy-related seeding, which became apparent six, nine, and 26 months after biopsy, respectively. In nine patients (3%) seeding was deemed to have occurred due to either biopsy or other interventions (as liver resection and radiofrequency ablative procedures); and five patients had seeding, which was assessed as a consequence of other invasive procedures than the biopsy. We were not able to determine the mean interval for seeding occurrence in all 17 patients as six of those had been exposed to several potential hazardous biopsies before abdominal wall metastases were detected. Fourteen patients had isolated liver metastases at the time of biopsy performing. Three patients had also metastases at other sites such as lungs, lymph nodes (above and below diaphragm). Six of 17 patients registered with seeding were exposed to CNB and in four patients only one attempt was recorded. In the post-biopsy course six of 17 (35%) patients were diagnosed with either carcinosis or ascites, or both. Resection of abdominal wall recurrences was performed in 13 of 17 patients (76%); one of those also received radiation due to recurrence and one patient was treated with radiation alone. Four seeded patients are still alive and currently receiving chemotherapy. Thirteen of 17 patients (76%) patients died from cancer-related progression, in particular due to liver metastases. Patients registered without seeding after biopsy had a shorter median overall survival time than those 17 patients with seeding 39 [95% confidence interval (CI), 32.9–45.1] months versus 70 [95% confidence interval (CI) 51.2–88.8] months. Fifty-one (18.3%) and 56 (20.1%) of all included CRC patients were identified with carcinosis and ascites, respectively, in the post-biopsy period. Almost two thirds (69%) of all patients died because of progression of liver metastases, and only six (3%) patients were registered with malignant intestinal obstruction as a cause of death.
Discussion
There is a growing interest for metastases biopsy in view of the observations that primary CRC and BC and their metastases are not always biologically identical [Citation1,Citation2]. At the same time when patients develop drug resistance, a small proportion could actually have a molecular transformation, which would be very important to know in terms of the next treatment, and this information can be obtained only through a tissue diagnosis. The purpose of this study was to estimate frequency of underexposed in the literature implantation seeding after percutaneous liver biopsy and whether this complication affected outcome. Other potential complications after liver biopsy, such as pain, bleeding, infection and puncture of other viscera have been reported previously [Citation3–6,Citation16,Citation17]. The strength of our study is the lack of selection bias within our institution because all biopsied patients in the specific period of time were initially included in the analysis. In total 100% follow-up was registered. Liver biopsies were performed for diagnosis confirmation, pretreatment staging for choice of treatment and/or as requirement for clinical trial. Decisions for liver biopsy were made on a case by case basis. High liver biopsy rate at our institution is a reflection of a dense follow-up program. To our knowledge, this is the largest analysis concerning seeding complication after liver biopsy in patients with CRC and the first one regarding patients with BC to date. The interesting observation is that no seeding was demonstrated in patients with BC where skin metastases are relatively common. In contrast, abdominal wall seeding was observed in patients with CRC where skin metastases are rare.
In the material presented herein we registered 6% patients with seeding and no negative impact on the course of the disease or shortening of survival was observed. On one hand, this is a relatively small fraction of patients in contrast to previous reports dealing exclusively with CRC seeding [Citation7–9]. On another hand, the estimated rate is surprisingly high compared to large surveys and institutional series [Citation3–5]. Due to the small number of patients, the survival data are not conclusive and should be viewed with caution. Despite lower seeding frequency compared with previous more recently published data our results confirmed that seeding risk was not negligible and should be considered properly in the decision of biopsy. Carcinomatosis and ascites incidence after liver biopsy in patients with BC and CRC was comparable to that reported in the literature [Citation18,Citation19]. Most patients died as a result of progression of the hepatic metastases. Only six (3%) patients of all patients with biopsy died because of intestinal obstruction.
Current literature provides only sparse material about seeding rate after percutaneous biopsy of CRC liver metastases and limited by small sample size. Ohlsson et al. retrospectively reported implantation metastases in five (10%) of 51 patients with CRC liver metastases. Median time interval to seeding occurrence was four months, ranging from two to 49 months. The authors recommend eluding biopsy use in curative candidates because of the observed harmful impact on survival in four of five patients [Citation7]. After retrospective review of patients who had undergone liver resection, Jones et al. were also worried about the negative influence of biopsy on survival. Forty patients had undergone preoperative biopsy by percutaneous techniques under radiological guidance and needle-track deposits were found in six (15%) [Citation8]. Furthermore, Rodgers et al. retrospectively found biopsy-track dissemination in seven (16%) of 43 patients independently of the type of biopsy, surgical versus radiological [Citation9]. Patients may develop abdominal wall metastases without ever having a US-guided biopsy done or a metastasis may develop at a site distant from the puncture site, and in the latter case almost always will the abdominal wall metastasis erroneously be attributed to the US-guided biopsy. This may account for some of the very high seeding frequency reports mentioned above. However, there is almost always a long time span of several months between the visual or palpatory appearance of a seeding metastasis and the US-guided biopsy that caused it, and thus it may be difficult to make a causal connection between the two. This fact may account for the very low frequency of seeding reported in large literature surveys and questionnaires and institutional studies, including a previously published, material of complications related to 8000 US-guided procedures performed at our institution wherein no incidence of seeding was encountered [Citation3–6].
There are numerous reports concerning needle tract seeding complication of breast biopsy in patients with BC. However, information on BC seeding after liver biopsy does not exist.
All mentioned publications are limited by small numbers of reviewed patients and of retrospective nature. The retrospective nature of our study did not seem to have a significant underestimation effect because of the all patients’ medical electronic records and regular diagnostics images were available and were assessed by both oncologists and intervention radiologists. We included all biopsies, performed at our institution in a specific period of time to eliminate exaggeration bias. The fact that some patients die because of cancer progression before seeding nodules were clinically detectable can impede accurate estimating. Thus, to minimize underestimation factor and to ensure adequate follow-up data we determined a minimum duration of follow-up after biopsy as three months based on published time interval to seeding occurrence ranging from two to 49 (median four) months. The majority (94.6%) of included patients received systemic drugs after biopsy that potentially could inhibit seeding incidence. Only tumors outside the liver parenchyma were regarded as seeding because it was hard to differentiate between real malignant cell seeding and progression, which obviously could undermine results. However, this weakness seems to outweigh the risk of overestimation effect and is balanced with including and categorizing all suspect cases.
Modern imaging techniques, particularly using US contrast can with confidence diagnose liver metastases, thus biopsy of surgically resectable liver metastases (seen on imaging) is not indicated anymore (in fact, is contraindicated specifically due to the risk of seeding). In contrast, biopsy is still indicated in the every case of doubt, if the patient is to be started chemo- or radiotherapy and/or, in case of possible obtained biopsy information can impact therapeutic decision. This is a routine practice at our institution; and indication for biopsy is being discussed scrupulously in every specific case, based exactly on the results of the present study. The best way to avoid seeding on is NOT to take the biopsy at all. Co-axial needle technique has been proposed for reducing the risk of seeding, however, a possible beneficial effect of this technique has never been demonstrated in randomized trials [Citation20]. Detection of circulating tumor DNA mutations by non-invasive blood collection, so-called liquid biopsy, is under active promising investigation and it is likely to be used as an alternative to tissue testing in the near future [Citation21].
Conclusion
Surprisingly, needle tract seeding following liver biopsy in BC patients is unlikely. Therefore, current recommendations regarding metastases biopsy should be continued without change in the clinical algorithm due to fear of seeding complication. Liver biopsy in CRC patients resulted in lower incidence (6%) seeding, comparing with previous published data. Albeit this risk cannot be considered negligible, it is, however, important to emphasize that seeding did not have any negative impact on the outcome. Thus, liver biopsy is justified when it is indicated for treatment decision; although indication for liver biopsy should be carefully considered in potential resectable CRC candidates, based upon presented data. Nevertheless, the data needs to be interpreted with caution due to the small number of patients.
Supplementary material available online
Table_4.docx
Download MS Word (44.1 KB)Acknowledgments
On behalf of the authors, I thank all the departments and collaborators for their contribution to the collection of patient data. I am grateful to director and staff from the Department of Oncology Herlev Hospital for their assistance and acceptance of this study. All authors declare that this submission is own work and has not been published before. All authors agree with the submission and the authors have declared no conflicts of interest.
References
- Vermaat JS, Nijman IJ, Koudijs MJ, Gerritse FL, Scherer SJ, Mokry M, et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: Implications for selection of patients for targeted treatment. Clin Cancer Res 2012;18:688–99.
- Aurilio G, Disalvatore D, Pruneri G, Bagnardi V, Viale G, Curigliano G, et al. A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. Eur J Cancer 2014;50:277–89.
- Weiss H, Duntsch U. [Complications of fine needle puncture. DEGUM survey II]. Ultraschall Med 1996;17:118–30.
- Fornari F, Civardi G, Cavanna L, Di SM, Rossi S, Sbolli G, et al. Complications of ultrasonically guided fine-needle abdominal biopsy. Results of a multicenter Italian study and review of the literature. The Cooperative Italian Study Group. Scand J Gastroenterol 1989;24:949–55.
- Weiss H. Metastases caused by fine needle puncture? Ultraschall Med 1989;10:147–51.
- Nolsoe C, Nielsen L, Torp-Pedersen S, Holm HH. Major complications and deaths due to interventional ultrasonography: A review of 8000 cases. J Clin Ultrasound 1990;18:179–84.
- Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg 2002;89:757–62.
- Jones OM, Rees M, John TG, Bygrave S, Plant G. Biopsy of resectable colorectal liver metastases causes tumour dissemination and adversely affects survival after liver resection. Br J Surg 2005;92:1165–8.
- Rodgers MS, Collinson R, Desai S, Stubbs RS, McCall JL. Risk of dissemination with biopsy of colorectal liver metastases. Dis Colon Rectum 2003;46:454–8.
- Metcalfe MS, Bridgewater FH, Mullin EJ, Maddern GJ. Useless and dangerous – fine needle aspiration of hepatic colorectal metastases. Br Med J 2004;328:507–8.
- Robertson EG, Baxter G. Tumour seeding following percutaneous needle biopsy: The real story! Clin Radiol 2011;66:1007–14.
- Struve-Christensen E. Iatrogenic dissemination of tumour cells. Dissemination of tumour cells along the needle track after percutaneous, transthoracic lung biopsy. Dan Med Bull 1978;25:82–7.
- Ryd W, Hagmar B, Eriksson O. Local tumour cell seeding by fine-needle aspiration biopsy. A semiquantitative study. Acta Pathol Microbiol Immunol Scand A 1983;91:17–21.
- Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol 2005;16:485–91.
- Smith EH. The hazards of fine-needle aspiration biopsy. Ultrasound Med Biol 1984;10:629–34.
- Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol 1986;2:165–73.
- McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology 1990;99:1396–400.
- Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol 2010;28:3271–7.
- van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol 2014;40:963–9.
- Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, et al. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol 2006;187:1184–7.
- Diaz LA Jr, Bardelli A. Liquid biopsies: Genotyping circulating tumor DNA. J Clin Oncol 2014;32:579–86.