Abstract
Background We characterized the incidence of central nervous system (CNS) involvement, risk factors and outcome in a large single institution dataset of peripheral T-cell lymphoma (PTCL).
Methods Retrospective review of the PTCL database at Memorial Sloan Kettering Cancer Center. We identified 231 patients with any subtype of PTCL between 1994–2011 with a minimum six months of follow-up or an event defined as relapse or death.
Results Histologies included peripheral T-cell lymphoma–not otherwise specified (PTCL–NOS) (31.6%), angioimmunoblastic (16.9%), anaplastic large cell lymphoma (ALCL), ALK- (12.1%), ALCL, ALK + (6.1%), extranodal NK/T-cell lymphoma (7.4%), adult T-cell leukemia/lymphoma (ATLL) (7.4%), and transformed mycosis fungoides (8.7%). Seventeen patients had CNS disease (7%). Fifteen had CNS involvement with PTCL and two had diffuse large B-cell lymphoma and glioblastoma. Median time to CNS involvement was 3.44 months (0.16–103.1). CNS prophylaxis was given to 24 patients (primarily intrathecal methotrexate). Rates of CNS involvement were not different in patients who received prophylaxis. Univariate analysis identified stage III–IV, bone marrow involvement, >1 extranodal site and ATLL as risk factors for CNS disease. On multivariate analysis, >1 extranodal site and international prognostic index (IPI) ≥ 3 were predictive for CNS involvement. The median survival of patients with CNS involvement was 2.63 months (0.10–75).
Conclusions Despite high relapse rates, PTCL, except ATLL, carries a low risk of CNS involvement. Prognosis with CNS involvement is poor and risk factors include: >1 extra nodal site and IPI ≥3.
The peripheral T-cell lymphomas (PTCL) account for approximately 10–15% of non-Hodgkin’s lymphomas (NHL) and are comprised of 23 different entities [Citation1,Citation2]. However, the most common subtypes: peripheral T-cell lymphoma–not otherwise specified (PTCL–NOS), angioimmunoblastic T-cell lymphoma (AITL), and ALK-negative anaplastic large cell lymphoma (ALK-negative ALCL), account for approximately 60% of cases and are the predominant entities seen in the US and Europe [Citation2]. Given the rarity of these disorders and lack of randomized control trials, therapeutic strategies have most often been borrowed from the management of aggressive B-cell lymphomas. However, when compared to the outcomes in B-cell lymphomas, such strategies result in a poorer prognosis.
While the role and benefits of central nervous system (CNS) prophylaxis remain controversial in aggressive lymphomas, there are guidelines to suggest when CNS prophylaxis should be considered in aggressive B-cell lymphomas [Citation3,Citation4]. Previous studies in diffuse large B-cell lymphoma (DLBCL) have demonstrated that the incidence of CNS involvement ranges from 2% to 10% [Citation5–12]. Patients with DLBCL who have testicular, epidural, paranasal sinus, or bone marrow (BM) with large cell lymphoma involvement, or greater than one extranodal site of disease and elevated lactate dehydrogenase (LDH) have been found to be at particularly high risk of CNS relapse and therefore are frequently recommended for CNS prophylaxis with intrathecal (IT) chemotherapy (methotrexate (MTX) and/or cytarabine) or systemic high dose MTX [Citation4–9]. In most of the large studies, the median time to CNS relapse is less than a year [Citation6,Citation8,Citation9,Citation13]. IT therapy has been shown to decrease the incidence of CNS relapse in lymphoblastic and Burkitt’s lymphoma from 78% to 19% in patients prior to the incorporation of rituximab [Citation8]. However, the role of CNS prophylaxis in DLBCL remains controversial [Citation7,Citation14].
There is little data regarding the incidence of CNS involvement in aggressive T-cell lymphomas and the use of CNS prophylaxis in aggressive T-cell lymphomas. The British Committee for Standards in Hematology recommends that guidelines regarding prophylaxis in aggressive B-cell lymphomas be extrapolated to aggressive T-cell lymphomas but does note that there is little data to support this [Citation3]. With regards to T-cell lymphomas, the National Comprehensive Cancer Network (NCCN) guidelines only recommend prophylaxis with IT chemotherapy for adult T-cell leukemia/lymphoma (ATLL).
Several retrospective series report CNS involvement in 5–9% of patients with PTCL [Citation15–17]. In a larger series from Korea describing 228 patients with PTCL excluding NK/T-cell lymphoma who did not receive CNS prophylaxis, the incidence of CNS relapse was 8.7%. Median time to CNS relapse was six months and those who developed CNS disease had inferior survival [Citation17]. In that series an increased LDH and involvement of the paranasal sinuses were predictive of CNS involvement. In a letter to the editor, Pro et al. reported a much lower risk of CNS relapse with a rate of 2.4% in patients with PTCL at MD Anderson Cancer Center. Of the patients who had CNS relapses, all had extranodal involvement and four had an elevated LDH [Citation18].
In this retrospective study we aimed to characterize the rate and nature of CNS involvement in 231 patients diagnosed with PTCL at a single institution from 1994 through 2011.
Methods
Patients
We reviewed our T-cell lymphoma database from 1994 to 2011 to include patients who were treated at our center at the time of their initial diagnosis and met all the following criteria: age 15 years old or above, a confirmed histological diagnosis of mature T-cell lymphoma based on the Revised European American Lymphoma (REAL), World Health Organization (WHO) 2003, or WHO 2008 classifications (based on what system was in use at the time of diagnosis), and at least six months follow-up or an event defined as relapse or death from any cause. We excluded indolent forms of cutaneous T-cell lymphomas including cutaneous anaplastic T-cell lymphoma, lymphomatoid papulosis and mycosis fungoides. Those with transformed mycosis fungoides were included. In all cases, the diagnosis was confirmed by pathologic review at Memorial Sloan Kettering Cancer Center (MSKCC).
We initially identified 273 patients of whom 42 patients were excluded from this analysis for: no adequate follow-up (N = 26), age under 15 (N = 2), diagnosis other than aggressive mature T-cell lymphoma (N = 14). Data collected included: age, gender, histologic subtype of T-cell lymphoma, human immunodeficiency virus (HIV) and human T lymphotropic virus-1 (HTLV-1) status, stage, number of extra nodal sites, sites of involvement including nasopharynx (NP), sinus, testes and bone marrow (BM), level of LDH, IPI and prognostic index of PTCL (PIT) – for detecting risk factors of CNS involvement.
Diagnosis of CNS disease
The diagnosis of CNS involvement was defined either pathologically or clinically. Pathologic confirmation of CNS involvement included identification of malignant cells in the cerebrospinal fluid (CSF) by cytology, flow cytometry and/or histologic identification of T-cell lymphoma on brain biopsy. Clinically diagnosed CNS involvement was based upon characteristic radiographic findings by magnetic resonance imaging (MRI), computed tomography (CT) or positron emission tomography (PET/CT) along with CNS-related symptoms sufficient for treating physicians to render the diagnosis. All imaging was done at our institution and standard clinical work-up including exclusion of infectious etiologies was performed to the extent possible in all cases.
Cases not meeting the above criteria were defined as having no CNS involvement.
CNS prophylaxis
Medical records were reviewed to ascertain CNS prophylaxis use. Subjects were coded as receiving CNS prophylaxis if in the absence of documented CNS disease they received at least one of the following as part of their initial therapy: IT MTX 12–15 mg, IT cytarabine 30 mg, and/or IV high dose MTX of at least 3 g/m2 [Citation3,Citation4,Citation19].
Statistical analysis
The primary outcome was the development of CNS involvement at any time from initial diagnosis. The secondary outcomes included: incidence of CNS involvement in various T-cell lymphoma histologies, time to CNS involvement (defined as time from the initial diagnosis of T-cell lymphoma until documented CNS involvement), median survival in patients with CNS involvement (defined as survival from the time of CNS disease until death from any cause), effect of CNS prophylaxis on the incidence of CNS involvement, and univariate and multivariate analysis of potential risk factors for CNS involvement including: age, histology, performance status (PS), stage, LDH level, >1 extra nodal site, BM involvement, IPI, and HTLV-1 status. The Kaplan-Meier method was used to create survival curves illustrating the time to CNS involvement from diagnosis. Univariate analyzes were performed with the log rank test to determine the impact of various variables. p-Values of <0.05 were considered significant. Factors found significant by univariate analysis were evaluated with multivariate analysis by Cox regression model. Fischer’s exact two-sided test was used to identify associations between variables. SPSS 19 IBM software was used to perform all analyzes.
Results
A total of 231 patients with PTCL were included in this retrospective analysis. The patients’ characteristics are shown in . The median age was 58 years (range 16–100) with 59.7% male. Histologies included PTCL–NOS (31.6%), AITL (16.9%), ALCL, ALK-negative (12.1%), ALCL, ALK positive (6.1%), extra nodal NK/T-cell lymphoma (ENKTCL), nasal type (7.4%), HTLV-1-associated ATLL (7.4%), hepatosplenic T-cell lymphoma (3.9%), transformed mycosis fungoides (8.7%) and others (6.1%). The median follow-up for the entire cohort was 4.9 years and the median overall survival for the entire cohort was 3.3 years.
Table I. Patients’ characteristics (231 patients).
Any CNS malignancy was found in 17 patients (7%) of subjects. Based on brain biopsy, two patients were found to have DLBCL (N = 1) and glioblastoma (N = 1) leaving 15 (6.5%) patients with pathologically confirmed or clinically diagnosed CNS involvement of T-cell lymphoma. Of these 15 cases with CNS involvement by T-cell lymphoma, eight patients were pathologically confirmed and seven patients were clinically confirmed. A pathologically confirmed diagnosis was made by positive cytology in seven cases and by suspicious cytology with a positive flow cytometry in one case. The clinical diagnosis was based on clinical symptoms with supporting imaging (brain CT or MRI) in five patients, on imaging and positive T-cell receptor gene rearrangement by polymerase chain reaction (PCR) in one case and on finding atypical cells in the CSF without confirmed flow or cytology in another case.
CNS disease typically presented early, with a median time from diagnosis of lymphoma to CNS involvement of 3.44 months (range 0.16–103.1 months) (). Four were diagnosed with CNS involvement at presentation and/or initial work-up prior to initiating therapy, an additional three were diagnosed during their initial chemotherapy, and eight in follow-up (at median of 7.85 months range 2.10–103.1 months). Of those who developed CNS disease during follow-up, three had an isolated CNS relapse while the other five had concurrent systemic relapse at the time. The median survival of patients after development of CNS involvement was 2.63 months (range 0.10–75.3 months).
Figure 1. A) Time to CNS relapse overall, B) Time to CNS relapse by histology, C) Time to CNS relapse by IPI, D) Time to CNS relapse by extranodal involvement.
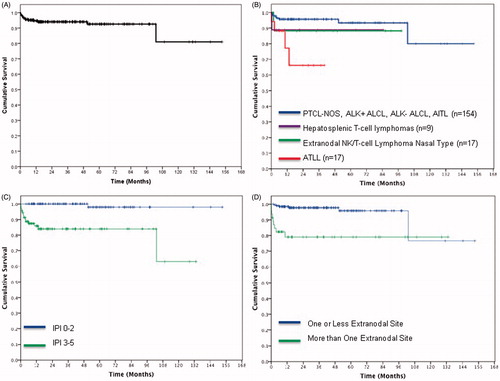
The histology of patients with CNS involvement include six patients with PTCL–NOS, four patients with ATLL, two patients with ENKTCL and one patient of each of the following: AITL, ALK-negative ALCL and hepatosplenic T-cell lymphoma (). Of those who developed CNS disease, risk factors at diagnosis included: high or high-intermediate IPI, 14/15 (93.3%); elevated LDH 12/15 (80%); and greater than one site of extra nodal disease, 9 of 15 (60%) (See ). None of the three HIV positive patients developed CNS disease.
By univariate analysis, four individual parameters were predictive for CNS involvement: stage III–IV (p = 0.033), BM involvement (p = 0.022), extra nodal involvement of more than one site (p < 0.001) and ATLL versus all other histologies 23.5% versus 6.4% (p = 0.001). By multivariate analysis, extra nodal involvement of more than one site was predictive of CNS disease (p = 0.004) (). High-intermediate and high IPI (IPI 3–5 & 4–5) were also found to be predictive for CNS involvement (p < 0.001) (See , ). Also, within the subgroup of patients with high IPI, extra nodal involvement of more than one site (p = 0.047) and ATLL (p = 0.014) were found to be significant risk factors for CNS disease.
Table II. Univariate and multivariate analysis of factors predictive of CNS involvement.
CNS prophylaxis was not applied in a standard fashion. Twenty-four (10.3%) patients received prophylaxis, most often IT MTX (N = 21). The grounds for using prophylaxis was documented in 20 patients and included: HTLV positive serology (N = 8), multiple extra nodal sites (N = 5), BM involvement (N = 3), high IPI (N = 3), and testis involvement (N = 1). The use of prophylaxis did not appear to reduce the incidence of CNS disease. In patients who received CNS prophylaxis, CNS involvement developed in 12.5% (3/24). In patients who did not receive prophylaxis, the incidence of CNS was 5.9% (12/204) (p = 0.194). In patients receiving CNS prophylaxis with high-intermediate and high IPI, the incidence of CNS disease was 25% (3 of 12 patients), while in patients who did not receive prophylaxis with the same IPI score, the incidence was 15.2% (11 of 79 patients) without any significant difference (p = 0.387).
Of note, eight of the 17 patients with HTLV1-associated lymphoma received prophylaxis. One patient was treated for CNS disease at diagnosis and eight did not receive CNS-directed therapy. In total four of the 17 patients with HTLV1+-associated disease developed CNS disease, of whom two had received prophylaxis and one was treated for CNS involvement at diagnosis.
Also, there was no significant difference in developing CNS disease between patients with low and low-intermediate IPI who received CNS prophylaxis (0/12) and those who did not (1/127) (p = 1.0). Furthermore, level of LDH did not influence the risk of CNS relapse whether patients received CNS prophylaxis or not.
Discussion
As T-cell lymphomas are an uncommon form of NHL, little has been reported regarding the incidence and risk factors for CNS involvement in PTCL. Prior retrospective series suggest that the rate of CNS involvement in PTCL ranges from 2% to 9% [Citation15–18]. One series from Korea found that presence of an elevated LDH and sinus involvement as risk factors for CNS involvement in PTCL. In their model, patients with zero, one or two risk factors, had an incidences of CNS involvement of 1.3%, 10.6% and 23.8%, respectively [Citation17]. In another retrospective study restricting the population to those with NK/T-cell lymphoma showed that an NK/T-cell lymphoma prognostic index, including the presence or absence of B symptoms, stage, elevated LDH and lymph node involvement, was the best predictor of the risk of CNS relapse [Citation20]. In the limited literature from North America the reported risk of CNS involvement in those with T-cell lymphoma appears much lower ranging from 2.4% to 3.6% [Citation16,Citation18]. In our series 6.5% (15/231) patients had CNS involvement at any point in their disease course. The strongest individual risk factors associated with developing CNS disease were advanced stage III–IV, BM involvement, extra nodal involvement of more than one site, and ATLL with rates of CNS involvement of 8.4%, 13%, 19.6%, and 23.5%, respectively. Subjects with high-intermediate or high IPI score of 3–5 also appear to be at elevated risk. The median time from diagnosis to CNS disease was 3.44 months (range 0.16–103.1) and the prognosis was poor with a median survival of less than 3 months form the time of CNS disease.
While it is known that CNS relapse is associated with a poor prognosis in both aggressive B- and T-cell lymphomas, the role of CNS prophylaxis remains somewhat unclear. There remains debate regarding whether to give CNS prophylaxis, to whom and the modality of prophylaxis (IT or systemic). Many DLBCL studies to determine the utility of CNS prophylaxis were performed in high-risk groups by analyzing potential risk factors. The use of IT MTX as prophylaxis is also controversial, and many studies in the post-rituximab era have questioned its efficacy [Citation5,Citation6,Citation11,Citation21]. Therefore, the NCCN recommends CNS prophylaxis in patients with DLBCL who have testicular, epidural, paranasal sinus, or BM with large cell lymphoma involvement, or greater than one extra nodal site of disease and elevated LDH [Citation4].
In the series from Korea, Yi et al. reported that the time to CNS relapse was 6.04 months [Citation17]. This was also shown in previous reports of DLBCL, with most of CNS events occurred between four and nine months after the initial diagnosis.
Development of CNS relapse has been shown to be associated with poor prognosis. In T-cell lymphomas, prior series have reported median survivals after diagnosis of CNS disease of approximately 1.0–7.0 months [Citation17,Citation18]. In our series, median survival after diagnosis of CNS relapse was 2.63 months.
In this study, the administration of CNS prophylaxis was not employed uniformly and the number of patients who were given CNS-directed prophylaxis was limited. Therefore, while the efficacy of CNS prophylaxis cannot be fully assessed in our series, the rate of CNS relapse was not significantly different in patients who received CNS prophylaxis compared to those who did not.
Nevertheless, within the subgroup of patients with high-intermediate and high IPI, extra nodal involvement of more than one site was found to be a significant risk factor for CNS disease. Unfortunately, we could not demonstrate that the use of CNS prophylaxis in high-risk groups, such as those with high IPI or high LDH, improves the effectiveness of CNS prophylaxis. However, given the low incidence of CNS relapse, even future studies may not be able to demonstrate this.
In conclusion, despite high relapse rates, PTCL, with the exception of ATLL, carries a relatively low risk of CNS involvement. Survival of patients with PTCL who develop CNS involvement remains poor. Based on our analysis risk factors that increase the risk of CNS relapse include: more than one extra nodal site and high-intermediate and high IPI.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Steven H, Swerdlow, EC, Nancy Lee H, Elaine S. Jaffe and Stefano A. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Pileri, Harald Stein, Jurgen Thiele, and James W. Vardiman, eds. Lyon, France: International Agency for Research on Cancer (IARC); 2008.
- Vose J, Armitage J, Weisenburger D, International TCLP. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol 2008;26:4124–30.
- Mcmillan A, Ardeshna KM, Cwynarski K, Lyttelton M, Mckay P, Montoto S. Guideline on the prevention of secondary central nervous system lymphoma: British Committee for Standards in Haematology. Br J Haematol 2013;163:168–81.
- National Comprehensive Cancer Network (NCCN). Non Hodgkin's Lymphoma Clinical Practice Guidelines in Oncology. (3.2014), 451. Retrieved from website: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf
- Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood 2009;113:3896–902.
- Feugier P, Virion JM, Tilly H, Haioun C, Marit G, Macro M, et al. Incidence and risk factors for central nervous system occurrence in elderly patients with diffuse large-B-cell lymphoma: Influence of rituximab. Ann Oncol 2004;15:129–33.
- Haioun C, Besson C, Lepage E, Thieblemont C, Simon D, Rose C, et al. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin's lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: A GELA study on 974 patients. Groupe d'Etudes des Lymphomes de l'Adulte. Ann Oncol 2000;11:685–90.
- Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte Hl. Central nervous system involvement following diagnosis of non-Hodgkin's lymphoma: A risk model. Ann Oncol 2002;13:1099–107.
- van Besien K, Ha CS, Murphy S, Mclaughlin P, Rodriguez A, Amin K, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood 1998;91:1178–84.
- Shimazu Y, Notohara K, Ueda Y. Diffuse large B-cell lymphoma with central nervous system relapse: Prognosis and risk factors according to retrospective analysis from a single-center experience. Int J Hematol 2009;89:577–83.
- Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: The impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol 2010;21:1046–52.
- Yamamoto W, Tomita N, Watanabe R, Hattori Y, Nakajima Y, Hyo R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol 2010;85:6–10.
- Herrlinger U, Glantz M, Schlegel U, Gisselbrecht C, Cavalli F. Should intra-cerebrospinal fluid prophylaxis be part of initial therapy for patients with non-Hodgkin lymphoma: What we know, and how we can find out more. Semin Oncol 2009;36:S25–34.
- Tomita N, Kodama F, Kanamori H, Motomura S, Ishigatsubo Y. Prophylactic intrathecal methotrexate and hydrocortisone reduces central nervous system recurrence and improves survival in aggressive non-hodgkin lymphoma. Cancer 2002;95:576–80.
- López-Guillermo A, Cid J, Salar A, López A, Montalbán C, Castrillo JM, et al. Peripheral T-cell lymphomas: Initial features, natural history, and prognostic factors in a series of 174 patients diagnosed according to the R.E.A.L. Classification. Ann Oncol 1998;9:849–55.
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol 2004;15:1467–75.
- Yi JH, Kim JH, Baek KK, Lim T, Lee DJ, Ahn YC, et al. Elevated LDH and paranasal sinus involvement are risk factors for central nervous system involvement in patients with peripheral T-cell lymphoma. Ann Oncol 2011;22:1636–43.
- Pro B, Perini G. Central nervous system prophylaxis in peripheral T-cell lymphoma. Blood 2010;115:5427
- Hill QA, Owen RG. CNS prophylaxis in lymphoma: Who to target and what therapy to use. Blood Rev.; 2006;20:319–32.
- Kim SJ, Oh SY, Hong JY, Chang MH, Lee DH, Huh J, et al. When do we need central nervous system prophylaxis in patients with extranodal NK/T-cell lymphoma, nasal type? Ann Oncol 2010;21:1058–63.
- Kumar A, Vanderplas A, Lacasce AS, Rodriguez MA, Crosby AL, Lepisto E, et al. Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: Findings from a large national database. Cancer 2012;118:2944–51.
