Abstract
Background: This study was aimed to assess the risk of breast cancer associated with exposure to insulin glargine in women with type 2 diabetes and evaluate whether the pattern of risk concurs with the hypothesized trend of an increase in risk with longer duration of use, taking into account previous cumulative exposure to other types of insulin.
Methods: We performed a restrospective cohort study (2002–2013) in the Clinical Practice Research Datalink among adult female patients with a first ever insulin prescription (n = 12 468). Time-dependent exposure measures were used to assess associations with duration of use of: (1) other insulin types before glargine was first prescribed (i.e. among switchers); and (2) of glargine during follow-up. Analyses were performed separately for insulin-naïve glargine users and patients switched to glargine. Cox proportional hazards models were used to derive p-trends, hazard ratios (HR) and 95% confidence intervals (CI) for breast cancer associated with glargine use.
Results: During 66 151 person years, 186 breast cancer cases occurred; 76 in glargine users (3.0/1000 years) and 110 in users of other insulins (2.7/1000 years). Among insulin-naïve women, no association with cumulative glargine use was observed (p-trend = 0.91), even after ≥5 years (HR = 1.06, 95% CI 0.48–2.33). Among switchers, a linear trend with years of prior exposure to other insulins was found (p-trend = 0.02). An increased risk was observed in glargine users with extensive (>3 years) past exposure to other insulins (HR = 3.17, 95% CI 1.28–7.84). A non-significant trend with cumulative glargine exposure was found among switchers (p-trend = 0.24).
Conclusions: Exposure to glargine was not associated with an increased breast cancer risk in insulin-naïve patients. Exposure to other insulins prior to the start of glargine appears to be relevant when studying breast cancer risk associated with glargine use.
In women, type 2 diabetes is associated with an increased risk of breast cancer [Citation1,Citation2]. In 2009, a number of observational studies emerged that linked the use of long-acting insulin glargine to an increased cancer incidence among women with type 2 diabetes, in particular breast cancer [Citation3–5]. Since then, treatment with insulin glargine has been studied intensively for its possible association with an increased breast cancer risk [Citation6,Citation7]. However, observational studies among insulin users are complicated by the fact that all insulins to some extent act as a growth stimulating factor [Citation8].
Human insulin acts as a growth promoting agent, stimulating breast cancer cell growth and inhibiting apoptosis in vitro [Citation9]. Differences in mitogenic potency between human insulin and insulin analogs on breast cancer cells have been shown in vitro [Citation8,Citation10]. Insulin glargine specifically appears to have an increased mitogenic potency [Citation8,Citation10], possibly related to its increased affinity for the insulin-like growth factor-1 receptor [Citation11]. Overall, results from cell studies indicate a cell growth stimulating effect, rather than a carcinogenic effect [Citation12]. Consequently, the risk of breast cancer is expected to increase with longer duration of exposure.
However, the majority of epidemiological studies conducted did not assess trends in breast cancer risk with duration of glargine use. Of the more detailed second generation studies, only five assessed cancer risk in relation to cumulative exposure measures [Citation13–17]. All of them evaluated duration of glargine use among insulin-naïve users separately and only one of the five studies observed a significant increased breast cancer risk associated with high cumulative exposure to glargine [Citation14]. However, all studies lacked sufficient follow-up to robustly estimate cancer risk beyond three years of cumulative duration of use [Citation13–17]. Therefore, further study of breast cancer risk with long-term glargine use (>3 years) remains necessary.
Of the two studies that included prevalent insulin users who switched to glargine, one observed an increased breast cancer risk after at least five years of cumulative glargine use [Citation13]. The other did not find an association with duration of use, but was unable to study effects of long-term glargine use due to a median duration of glargine use of 1.2 years [Citation14]. Both studies determined exposure to glargine at baseline (intention-to-treat) and were limited by left-truncated data, which resulted in misclassification of duration of exposure and potential underestimation of past exposure to other insulins prior to the switch to glargine. Moreover, neither study was able to determine how the duration of non-glargine insulin use before cohort entry modifies the effect of glargine use.
The aim of our study was to assess the risk of breast cancer associated with exposure to insulin glargine in women with type 2 diabetes and evaluate whether the pattern of risk concurs with the hypothesized trend of an increase in risk with longer duration of use, taking into account previous cumulative exposure to other types of insulin.
Methods
Source of data
Data were obtained from the Clinical Practice Research Datalink (CPRD), which comprises electronic medical records from British general practitioners since 1987 [Citation18]. The accuracy and completeness of CPRD data have been well validated in previous studies [Citation19]. Currently, CPRD includes approximately 7% of the total UK population [Citation18]. The protocol of this study was approved by CPRD’s Independent Scientific Advisory Committee.
Study population
For this retrospective cohort study, we used a ‘new user’ design with incident insulin users. All women (≥18 years) with at least one prescription for any type of insulin in CPRD during the inclusion period were eligible. To ensure a minimal follow-up period of approximately three years between cohort entry and the end of data collection (1 October 2013), the inclusion period stretched from 1 September 2002 – the marketing date of glargine in the UK – to 31 December 2010. The index date was defined as the date of the first recorded prescription for any type of insulin within the inclusion period. On the index date, all subjects were required to have at least one year of up-to-standard patient history in CPRD without any recorded history of insulin use to improve the validity of the ‘new user’ design.
Patients considered to have type 1 diabetes were excluded. These were patients without any use of non-insulin antidiabetic drugs (NIADs) in the year prior to cohort entry who: 1) had a recorded diagnosis for type 1 diabetes before cohort entry; or 2) were under 30 years of age at cohort entry. Subjects with a history of breast cancer at baseline were also excluded. All subjects were followed from the index date until the outcome of interest, end of data collection, date of migration out of the CPRD population, or death, whichever came first.
Exposure to insulins
We used a time-dependent design () to define exposure. For all patients, the follow-up period after the index date was divided into discrete 30-day intervals. Exposure to glargine (‘any use’) was then defined as a prescription for glargine on the start date or at any time before the start of each interval. Patients with a prescription for non-glargine insulin (i.e. any insulin type except glargine) at cohort entry could become exposed during follow-up if a prescription for glargine was recorded (‘switchers’). Current exposure to glargine and non-glargine insulins was defined as a prescription on the start date or in the three months prior to the start of each 30-day interval.
Figure 1. Schematic representation of exposure measures used. The basic time-dependent design considers a patient exposed from the first prescription of glargine onward. Cumulative exposure measures were included as a refinement, where patients are stratified by (a) cumulative exposure to insulin glargine during follow-up, (b) duration of exposure to other insulin types before the start of glargine treatment, and (c) stratified to insulin-naïve starters of glargine and patients switched to glargine.
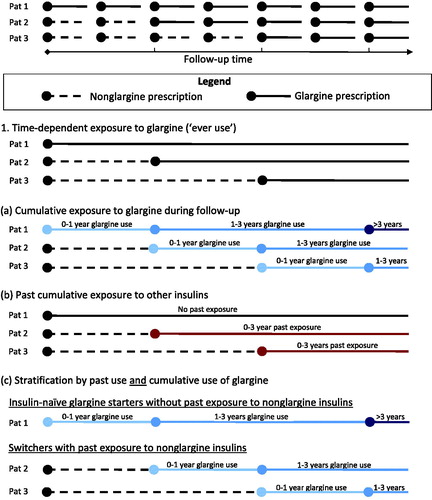
In a stepwise manner, we added time-dependent cumulative measures for duration of use of: (1) glargine during follow-up; and (2) non-glargine insulins before the initiation of glargine therapy. In a final model (3), we studied breast cancer risk associated with cumulative duration of glargine use separately among insulin-naïve glargine users and users of glargine with prior use of other insulin types (). Cumulative duration of use calculations were based on the number of days of ‘current exposure’.
Duration of use of glargine during follow-up was determined at the start of each 30-day interval and classified as ‘0–1 years’, ‘1–3 years’, or ‘>3 years’ (), based on the total number of days of current exposure to glargine. Consequently, cumulative exposure to glargine could only increase or remain stable over time.
In switchers, cumulative number of days of past exposure to non-glargine insulins was calculated at the start date of glargine treatment. Here, we differentiated between insulin-naïve patients at the start of glargine treatment, and switchers with a cumulative duration of past exposure to non-glargine insulins of ‘0–3 years’ and ‘>3 years’ ().
In our final model, we performed separate analyses regarding associations with duration of glargine exposure during follow-up among insulin-naïve patients and prevalent insulin users switched to glargine (). In all models, we quantified the risk of breast cancer associated with glargine use as compared to ‘never use’ of glargine; as the study was performed among insulin users, person time on glargine was compared to person time on other insulins.
Study outcome
All subjects were followed up for the occurrence of a first medical diagnosis of breast cancer in CPRD (see Supplemental Table A for the used medical codes, available online at http://www.informahealthcare.com). Completeness of case ascertainment for breast cancer in CPRD is high as compared to the national cancer registry data [Citation20]. A recent study found a concordance rate of 89.8% with cancer registries and a subsequent 6.4% of the records were in agreement with hospital records or death certificates [Citation20].
Covariates
Models were adjusted for potential confounders in a time-dependent manner. Age was determined as the year difference between calendar year and year of birth at the start of each 30-day interval. A history of cancer other than breast cancer (or non-melanoma skin cancer) and oophorectomy was determined as a medical diagnosis at any time before the start of each interval. Smoking status (yes or no) and alcohol use (yes or no) were determined at cohort entry and subsequently updated during follow-up at the start of each interval. Current use of comedication (i.e. hormone replacement therapy, statins, metformin, sulfonylureas, and glitazones) was determined as a prescription in the past 180 days prior to the start of each interval. For body mass index (BMI) and HbA1c, the most recent record before the start of follow-up was used to classify patients at baseline. Subsequently, obesity (BMI ≥30 kg/m2) and increased HbA1c level (>75 mmol/mol) were determined based on the most recent measurement at the start of each interval. We used stepwise model building for adjustment for potential confounders. In Model 1, we adjusted for all potential confounders, while in Model 2 we performed additional adjustment for number of years of past exposure to non-glargine insulins before the start of glargine as a continuous variable, to adjust for the potential effect of past exposure to non-glargine insulins on the association between glargine use and breast cancer risk among switchers. We evaluated the linearity assumption by adding a squared term to the model, together with the continuous variable.
Statistical analysis
Multivariate Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% confidence intervals (CI) of breast cancer associated with the use of glargine, with survival time in 30-day intervals as the time variable. For all models, ‘never use’ of glargine was used as the reference category. In addition to the analyses stratified by categories of cumulative duration of use, we performed p-trend analyses, where cumulative exposure to insulins was included as a continuous variable.
In a sensitivity analysis, an extended category of cumulative duration of glargine exposure was added – ‘0–1 years’, ‘1–3 years’, or ‘3–5 years’, and ‘>5 years’ – when study power was sufficient. In a separate analysis, the cumulative duration-response effect was studied among glargine users independently. Here, patients started on glargine were censored at the time a different insulin type was prescribed. In this sensitivity analysis, the lowest category of cumulative exposure was taken as the reference.
All data management and statistical analyses (PROC PHREG) were conducted using SAS 9.2 (SAS Institute Inc, Cary, NC, USA).
Results
For this study, we selected 12 468 female incident insulin users for our final study cohort (Supplemental Figure A, available online at http://www.informahealthcare.com). Baseline characteristics of new users of insulin who received a first prescription for glargine (n = 3858) or non-glargine insulins (n = 8610) are shown in . The median duration of follow-up was comparable between the glargine and non-glargine starters (5.0 vs. 5.1 years), as was the median duration of insulin exposure during follow-up; 2.6 years of glargine use among glargine starters, and 3.0 years of non-glargine insulin use among non-glargine starters. Glargine starters were in general older at baseline (median age of 66 vs. 61 years). Of the non-glargine starters, the majority received a first prescription for insulin aspart (44.6%) or human insulin (39.4%). Glargine starters in general received NIADs in the year prior to baseline (95.3%).
Table 1. Baseline characteristics of incident insulin users started on insulin glargine, non-glargine insulin, or both.
Risk of breast cancer
During a total follow-up of 66 151 person years, 186 breast cancer cases occurred. Of these, 76 occurred in patients after exposure to glargine (3.0 per 1000 person years), and 110 in patients never exposed to glargine (2.7 per 1000 person years). In our model adjusted for potential confounders (Model 1), no discernible increase in breast cancer risk was associated with ‘ever use’ of glargine (HR 1.06, 95% CI 0.79–1.44), as compared to ‘never use’ (). When adjusted for years of exposure to other insulins before the start of glargine treatment (Model 2), no risk difference was observed (HR 0.98, 95% CI 0.72–1.35).
Table 2. Hazard ratios for breast cancer associated with the use of insulin glargine, stratified by cumulative duration of glargine use during follow-up and by cumulative exposure to other insulins before the initiation of glargine therapy.
Cumulative exposure measures
Stratification by cumulative duration of exposure to glargine during follow-up (, ) did not show an association with breast cancer risk (p-trend = 0.83 in Model 1). Even when cumulative glargine use of over five years was modeled as a separate category (sensitivity analysis), no significant difference in risk was observed (HR 1.26, 95% CI 0.64–2.47 in Model 1).
Among switchers, stratification by prior exposure to non-glargine insulins at the start of glargine treatment (, ) showed a linear trend with increasing years of prior exposure to non-glargine insulins (p-trend = 0.02 in Model 1). Here, a significant three-fold increase in breast cancer risk was observed in switchers with a history of non-glargine insulin use of more than three years, as compared to women never exposed to glargine (HR 3.17, 95% CI 1.28–7.84).
Among insulin-naïve women (, ), no increased breast cancer risk was associated with ‘ever use’ of glargine (HR 0.99, 95% CI 0.71–1.37). Also, no trend with cumulative duration of glargine exposure was observed (p-trend = 0.91, HR = 0.99, 95% CI 0.90–1.10 per additional year of exposure). After additional stratification (sensitivity analysis), no increased breast cancer risk was associated with ≥5 years of cumulative exposure (HR = 1.06, 95% CI 0.48–2.33).
Table 3. Hazard ratios for breast cancer associated with the use of insulin glargine, among insulin-naïve glargine users and glargine users with prior exposure to other insulins, stratified by categories of cumulative glargine use and adjusted for use of other insulins before the start of glargine.
Among switchers, a slight, non-significantly increased breast cancer risk was associated with ‘ever use’ of glargine (HR = 1.40, 95% CI 0.83–2.34). Moreover, a non-significant trend was observed with cumulative number of years of glargine exposure (p-trend = 0.24, HR = 1.10, 95% CI 0.94–1.29 per additional year of exposure in Model 1). Adjustment for number of years of exposure to non-glargine insulins before the start of glargine treatment (Model 2) resulted in noticeable reductions in risk estimates. The HR for ‘ever use’ of glargine in switchers was reduced to 0.97 (95% CI 0.47–1.99), while the HR for the highest category of cumulative glargine exposure was reduced from 1.58 (95% CI 0.63–3.94) to 1.14 (95% CI 0.42–3.09) (). In addition, the non-significant trend with cumulative number of years of glargine exposure disappeared after adjustment for cumulative number of years of exposure to non-glargine insulins (p-trend = 0.99, HR = 1.00, 95% CI 0.91–1.10 per additional year of exposure, Model 2 (not shown)). In this model, a significant trend was observed with number of years of prior non-glargine insulin exposure (p-trend = 0.02, HR = 1.24, 95% CI 1.03–1.50 per additional year of exposure, Model 2) (not shown).
Results from our sensitivity analysis that censored glargine insulin users if any other type of insulin was initiated, also showed no trend with cumulative duration of use (Supplemental Table A).
Discussion
In this cohort study among women with type 2 diabetes newly started on insulin, glargine use was not associated with an increased risk of breast cancer after a median follow-up of five years as compared to use of other insulins. However, a difference in breast cancer risk between insulin-naïve new users of glargine and women who switched to glargine after having used other types of insulin was observed. More specifically, no association between glargine use (either in general or with cumulative use) and breast cancer risk was seen among insulin-naïve new users of glargine, even after five cumulative years of glargine exposure. In contrast, a non-significant increase in breast cancer risk was found among patients who switched to glargine, depending on the number of years of past insulin use. That is, a significant trend was observed for each additional year of non-glargine exposure before the start of glargine treatment.
Our results regarding insulin-naïve patients (i.e. without prior exposure to other insulins) are in agreement with those from most observational studies that used cumulative exposure measures. In the previous studies, four out of five did not show an association between cumulative duration of glargine use and breast cancer risk among insulin-naïve starters of glargine [Citation13,Citation15–17]. The study by Habel et al. (2013) did report an increased breast cancer risk associated with extended duration (≥2 years) of use (HR = 1.6, 95% CI 1.0–2.8) [Citation14]. This result might be a chance finding, as with a median duration of glargine use of 1.2 years they were unable to assess patterns of risk with longer duration of use. In fact, all previous studies among insulin-naïve glargine users were limited by insufficient study power to robustly estimate effects of long-standing (>3 years) glargine exposure. Moreover, our results are also in line with those from clinical trials among new users of insulin that consistently showed no increased cancer risk associated with glargine exposure [Citation21–23]. The ORIGIN trial, with a median follow-up of 6.2 years, found no increased risk of breast cancer among patients assigned to glargine versus standard care. However, with only 28 breast cancer cases in both treatment arms, study power was limited [Citation22].
Among patients switched to glargine after having used other insulins, we observed a non-significant increase in risk associated with glargine use. This result is in line with that of Suissa et al. (2011) who reported a significant risk increase associated with glargine use after five years or more among switchers [Citation13]. However, the 2.7-fold risk increase (95% CI 1.1–6.5) found in their study is much larger than the one observed in our study. Conversely, the only other study that included switchers, found no increased risk associated with duration of glargine use, but was limited by a relatively short follow-up for glargine users (median of 2.3 years) [Citation14]. In our study, we observed that breast cancer risk increased with each added year of non-glargine insulin use before the start of glargine (i.e. effect modification). Neither of the previous studies assessed the effect of past insulin exposure on the association between glargine use and breast cancer risk. Suissa et al. are thus far the only ones to acknowledge that duration of insulin use before the start of glargine use should be taken into account. However, by matching on duration of past use, the amount of a potential effect was not measured.
Extensive past use (≥3 years) of non-glargine insulins was associated with a three-fold increase in breast cancer risk among patients switched to glargine. However, when considering latency periods and the longer duration of type 2 diabetes and treatment thereof [Citation24], it is impossible to attribute this excess risk among switchers to a single factor (i.e. glargine use). Nonetheless, this result sheds some light on the dynamics linked to the apparent difference in breast cancer risk seen in insulin-naïve patients started on glargine and switchers with past exposure to other insulins. The importance of taken prior use of other insulins into account was demonstrated by the noticeable reduction in all risk estimates for breast cancer associated with glargine use after adjustment for the number of years previously exposed to other insulins.
Alternative explanations for the difference in breast cancer risk associated with glargine use between insulin-naïve starters of glargine and switchers may entail that total duration of insulin use (or diabetes duration), rather than exposure to any particular insulin type, is associated with an increased risk of breast cancer. In addition, patients switched to glargine may differ from insulin-naïve patients started on glargine. If glargine is used as an add-on in prevalent insulin users with poorly controlled blood glucose levels, a possible variation in background risk might be introduced. Such a dynamic could lead to channeling and potential protopathic bias among switchers. This alternative hypothesis might be evaluated in future studies.
Major strengths of our study include the use of time-dependent exposure measures based on prescription data to determine insulin exposure. This definition minimizes exposure misclassification and more accurately reflects real exposure than time since the start of follow-up, as used in previous studies. In addition, as only incident insulin users (≥1 year without any insulin use at baseline) were included, we had comprehensive information on insulin use for patients included in the cohort. In patients who switched to glargine during follow-up, we were able to determine the effect of past insulin use on the association between glargine use and breast cancer risk. To our knowledge, we are the first to assess this effect. Furthermore, we had three additional years of follow-up as compared to the study by Suissa et al. and were thereby able to determine breast cancer risk estimates for long-standing glargine use in insulin-naïve patients.
Several limitations of our study should be noted as well. First of all, the comparator consisted of all other (non-glargine) insulins. This category included both short- and long-acting insulins, resulting in a heterogeneous reference group. However, it can be regarded as a relevant reference group when you want to assess glargine associated risks versus the other treatment options available. Conversely, this approach did not allow for direct comparisons between long-acting insulin types. Second, our models did not account for any additional effects of combined use of both glargine and non-glargine insulins. Ideally, combined use should be considered as a separate category. Also, we were unable to make direct comparisons between glargine and non-glargine insulin users with the same duration of exposure. Third, we did not take latency into account, as follow-up time was insufficient to incorporate a sensible latency period for breast cancer. Fourth, as the cohort was restricted to new users of insulin at cohort entry, study power to analyze effects with cumulative duration of use was limited for the group of switchers, hindering further stratification by strata of cumulative years of past non-glargine insulin use. Fifth, as CPRD covers a dynamic patient population (i.e. ∼7% of the total UK population), patients who transfer to a general practitioner who does not provide data to CPRD are lost to follow-up. Finally, when fitting several exposure models on the same data, focus should not be on individual significant results, but the total of analyses should be seen in perspective and interpreted together [Citation25].
In conclusion, exposure to insulin glargine did not appear to be associated with an increased breast cancer risk in insulin-naïve patients. Our results, however, do indicate an association between glargine use and breast cancer among patients previously treated with other insulins before the start of glargine. Glargine use in patients with extensive past exposure to other insulin types was associated with a three-fold increased risk. Therefore, observational studies need to take past exposure to other insulins into account when studying breast cancer risk associated with glargine use. Future studies should consider whether this excess risk of breast cancer observed in patients switched to glargine is caused by protopathic bias.
Online-Only_Supplemental_File.docx
Download MS Word (40.6 KB)Disclosure statement
The research leading to the results of this study has received funding from the European Community’s Seventh Framework Program (FP-7) under grant agreement number 282526, the CARING project. The funding source had no role in study design, data collection, data analysis, data interpretation or writing of the report.
The Department of Pharmacoepidemiology and Clinical Pharmacology employing authors Paul J. H. L. Peeters, Maroes T. Bazelier and Frank de Vries received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private–public-funded Top Institute Pharma (http://www.tipharma.nl; includes co-funding from universities, government and industry), the Dutch Medicines Evaluation Board and the Dutch Ministry of Health.
Hubert G. M. Leufkens is employed by Utrecht University as professor conducting research under the umbrella of the WHO Collaborating Center for pharmaceutical policy and regulation. This Center receives no direct funding or donations from private parties, including pharma industry. Research funding from public-private partnerships, e.g. IMI, TI Pharma (www.tipharma.nl) is accepted under the condition that no company-specific product or company related study is conducted. The Center has received unrestricted research funding from public sources, e.g. Netherlands Organization for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), EU 7th Framework Program (FP7), Dutch Medicines Evaluation Board (MEB), and Dutch Ministry of Health.
Anssi Auvinen is employed by the University of Tampere as professor and has no personal or financial conflict of interest to report.
Tjeerd P. van Staa has participated in expert meetings with Glaxo Smith Kline and Boehringer and has provided methodological advice to Laser (all unrelated to the subject of this manuscript).
Marie L. De Bruin is employed by Utrecht University as a senior researcher conducting research in collaboration with the WHO Collaborating Center for pharmaceutical policy and regulation. This Center receives no direct funding or donations from private parties, including the pharma industry. Research funding from public-private partnerships, e.g. IMI and TI Pharma (http://www.tipharma.nl) is accepted under the condition that no company-specific product or company related study is conducted. The Center has received unrestricted research funding from public sources, e.g. Netherlands Organization for Health Research and Development (ZonMW), the Dutch Health Care Insurance Board (CVZ), EU 7th Framework Program (FP7), Dutch Medicines Evaluation Board (MEB) and Dutch Ministry of Health.
None of the abovementioned funding sources had a role in the design, conduct, analysis, or reporting of this study.
References
- Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies BMJ 2015;350:g7607.
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 2007;121:856–62.
- Colhoun HM. SDRN Epidemiology Group. Use of insulin glargine and cancer incidence in scotland: a study from the scottish diabetes research network epidemiology group. Diabetologia 2009;52:1755–65.
- Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjornsdottir S, Steineck G. Insulin glargine use and short-term incidence of malignancies-a population-based follow-up study in sweden. Diabetologia 2009;52:1745–54.
- Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia 2009;52:1732–44.
- Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, et al. Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf 2013;8:333–48.
- Bronsveld HK, Ter Braak B, Karlstad O, Vestergaard P, Starup-Linde J, Bazelier MT, et al. Treatment with insulin (analogues) and breast cancer risk in diabetics; a systematic review and meta-analysis of in vitro, animal and human evidence. Breast Cancer Res 2015;17:100. DOI: 10.1186/s13058-015-0611-2.
- Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, et al. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000;49:999–1005.
- Osborne CK, Bolan G, Monaco ME, Lippman ME. Hormone responsive human breast cancer in long-term tissue culture: Effect of insulin. Proc Natl Acad Sci USA1976;73:4536–40.
- Staiger K, Hennige AM, Staiger H, Haring HU, Kellerer M. Comparison of the mitogenic potency of regular human insulin and its analogue glargine in normal and transformed human breast epithelial cells. Horm Metab Res 2007;39:65–7.
- Weinstein D, Simon M, Yehezkel E, Laron Z, Werner H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab Res Rev 2009;25:41–9.
- Home P. Insulin therapy and cancer. Diabetes Care 2013;36Suppl:S240–4.
- Suissa S, Azoulay L, Dell'aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia 2011;54:2254–62.
- Habel LA, Danforth KN, Quesenberry CP, Capra A, Van Den Eeden SK, Weiss NS, et al. Cohort study of insulin glargine and risk of breast, prostate, and colorectal cancer among patients with diabetes. Diabetes Care 2013;36:3953–60.
- Sturmer T, Marquis MA, Zhou H, Meigs JB, Lim S, Blonde L, et al. Cancer incidence among those initiating insulin therapy with glargine versus human NPH insulin. Diabetes Care 2013;36:3517–25.
- Fagot JP, Blotiere PO, Ricordeau P, Weill A, Alla F, Allemand H. Does insulin glargine increase the risk of cancer compared with other basal insulins?: A french nationwide cohort study based on national administrative databases. Diabetes Care 2013;36:294–301.
- Lim S, Stember KG, He W, Bianca PC, Yelibi C, Marquis A, et al. Electronic medical record cancer incidence over six years comparing new users of glargine with new users of NPH insulin. PLoS One 2014;9:e109433.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical practice research datalink (CPRD). Int J Epidemiol 2015;44:827–36.
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the general practice research database: a systematic review. Br J Clin Pharmacol 2010; Jan;69:4–14.
- Boggon R, van Staa TP, Chapman M, Gallagher AM, Hammad TA, Richards MA. Cancer recording and mortality in the general practice research database and linked cancer registries. Pharmacoepidemiol Drug Saf 2013;22:168–75.
- Home PD, Lagarenne P. Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Diabetologia 2009;52:2499–506.
- ORIGIN Trial Investigators, Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med 2012;367:319–28.
- Rosenstock J, Fonseca V, McGill JB, Riddle M, Halle JP, Hramiak I, et al. Similar risk of malignancy with insulin glargine and neutral protamine hagedorn (NPH) insulin in patients with type 2 diabetes: findings from a 5 year randomised, open-label study. Diabetologia 2009;52:1971–3.
- Suissa S, Azoulay L. Metformin and the risk of cancer: Time-related biases in observational studies. Diabetes Care 2012;35:2665–73.
- Patel CJ, Ioannidis JP. Placing epidemiological results in the context of multiplicity and typical correlations of exposures. J Epidemiol Community Health 2014;68:1096–100.
