Abstract
Background: Osteosarcomas arising in the proximal femur, humerus, and tibia appear to have poorer outcomes than those arising in distal long bones. However, the strength of this association is uncertain, particularly in light of other prognostic factors. Therefore, this retrospective cohort study was performed to compare patient outcomes between proximal and distal tumor location within extremity long bones. Material and methods: A total of 153 patients with conventional high-grade osteosarcoma of the extremity long bones, pelvis, or axial skeleton who had undergone neoadjuvant chemotherapy and surgical resection between 1985 and 2010 were identified in the Surgical Pathology files at Vanderbilt Medical Center. Effect of anatomic location within a proximal long bone was assessed using multivariable Cox proportional hazard regression. Results: Proximal tumor location was a strong predictor of poor prognosis in univariate survival analysis. Multivariate regression analysis showed that after controlling for American Joint Committee on Cancer (AJCC) stage, histologic response to chemotherapy, surgical resection margin status, and histologic type, location in the proximal femur, tibia, and humerus were independent risk factors for death due to osteosarcoma, but not event-free survival. Conclusion: Osteosarcomas of the proximal extremity long bones are associated with decreased disease-specific survival compared to tumors of the distal long bones, even after accounting for other key prognostic covariates.
The prognostic impact of osteosarcoma arising in the proximal aspects of the long bones of the extremities has not been studied extensively. Tumors arising in the proximal femur and humerus appear to have worse outcomes. However, the proximal tibia was considered as a distal site of origin in prior studies [Citation1–4]. Other studies show no significant effect of proximal location [Citation5,Citation6] or attribute the adverse outcomes of proximal tumors to larger tumor size or decreased response to adjuvant chemotherapy [Citation7,Citation8]. Therefore, this retrospective cohort study was performed to define the relationship between tumor location within long bones of the extremities and patient outcomes.
Material and methods
The Surgical Pathology files at Vanderbilt University Medical Center were searched for patients with conventional high-grade osteosarcoma who had undergone neoadjuvant chemotherapy and surgical resection between 1985 and 2010. Diaphyseal tumors (n = 7) and acral tumors (n = 2) were excluded. A total of 153 patients who met these inclusion criteria were identified. Pathology reports were reviewed and the anatomic location and size of the tumor, histologic diagnosis, extent of tumor necrosis, American Joint Committee on Cancer (AJCC) stage [Citation9], presence of pathologic fracture, and the status of surgical resection margins were recorded.
H&E-stained slides were reviewed to confirm histologic type, extent of chemotherapy-induced tumor necrosis, and resection margin status. The extent of tumor necrosis was recorded as a percentage of total area of tumor examined [Citation10]. Clinical data (patient age at diagnosis, sex, chemotherapy dates and regimens, dates of surgery, local recurrence, distant metastasis, and death, as well as cause of death) were abstracted from electronic medical records. Osteosarcomas were considered secondary if they arose in association with prior retinoblastoma (n = 3), prior irradiation therapy (n = 2), bone infarct (n = 1), or Paget’s disease (n = 1).
The study protocol was approved by the Vanderbilt University Institutional Review Board (#120193; 02/07/2012); a waiver of informed consent was obtained.
Statistical analysis
Categorical variables were compared among study groups using Fisher’s exact test; continuous variables were compared using one-way ANOVA after log transformation. Univariate survival analysis was performed using Cox proportional hazard regression. Tumor size was missing for 14 cases, so missing values were imputed by partial means matching (n = 50). All variables were included in initial multivariate models; variables were then excluded in step-wise fashion in order of increasing Z-score until likelihood ratio tests showed that all remaining variables were contributory to the final regression model. All tests were two-sided with α = 0.05. All statistical analyses were performed using Stata v13.1 (StataCorp, College Station, TX, USA).
Results
Patient cohort
Of 153 patients who met inclusion criteria, 72 (47%) had tumors arising in the distal femur, tibia, humerus, or fibula, 54 (35%) had tumors involving the proximal aspects of these bones, and 27 (18%) had tumors of the pelvis (n = 10) or axial skeleton (n = 17). Patients who were censored at the time of last follow-up were followed for a median of 9 years (range 1 day–28 years). Sixty-six (43%) patients died of disease a median of 1.8 years after surgical resection of tumor (range 3 months–10.6 years). There were 85 events (death due to progressive disease or distant metastasis) a median 1.1 years after resection (range 1 month–26.6 years). Clinicopathologic characteristics of patients with distal tumors, proximal tumors, or axial or pelvic tumors are presented in . Proximal osteosarcomas were more common in the humerus (94%), tibia (74%), and fibula (60%) than the femur, where only 8% were proximally located. In addition, fewer proximal osteosarcomas showed good histologic response to neoadjuvant chemotherapy than did tumors arising in the distal epiphyses (57% vs. 76%). The rate of positive surgical resection margins in distal and proximal tumors was similar, whereas axial or pelvic tumors often had positive resection margins.
Table 1. Clinicopathologic characteristics of the Vanderbilt osteosarcoma cohort.
Disease-free and event-free survival
As expected, AJCC Stage IV disease, poor chemotherapy response, and positive surgical resection margins were adverse prognostic factors for disease- and event-free survival by univariate Cox proportional hazard regression (). In addition, location in the proximal humerus, proximal femur, pelvis, or axial skeleton was associated with increased hazard ratios for disease-free survival when compared to the distal femur. Multivariate regression analysis revealed that after controlling for AJCC Stage, histologic response to chemotherapy, resection margin status, histologic type, and secondary-type osteosarcoma, tumors of proximal long bones showed hazard ratios similar to those of axial and pelvic osteosarcomas for both disease-specific and event-free survival (). Although location within proximal long bones was an independent predictor of shorter disease-specific survival (), event-free survival hazard ratios for the proximal femur or proximal tibia did not reach statistical significance ().
Figure 1. Kaplan-Meier disease-specific survival curves for non-metastatic (AJCC Stage IIA and IIB) osteosarcomas arising in the proximal long bones (dashed line), distal long bones (solid line), or axial/pelvic skeleton (dotted line).
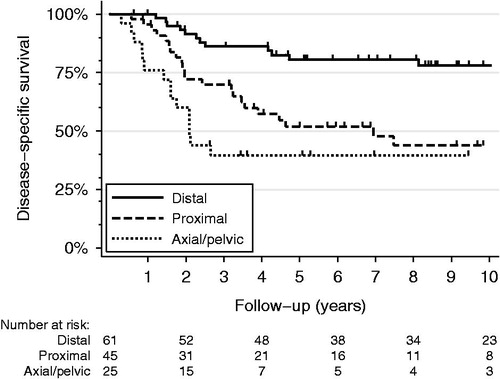
Figure 2. Kaplan-Meier event-free survival curves for non-metastatic (AJCC Stage IIA and IIB) osteosarcomas arising in the proximal long bones (dashed line), distal long bones (solid line), or axial/pelvic skeleton (dotted line).
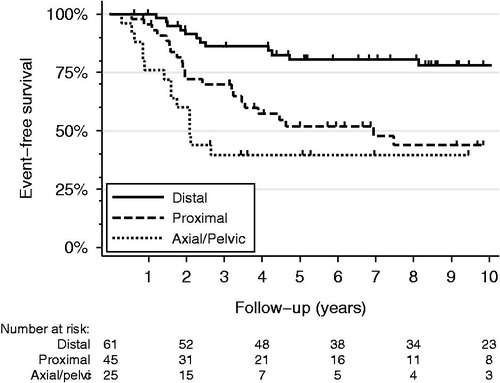
Table 2. Univariate disease-specific and event-free survival analysis (n = 153).
Table 3. Multivariate disease-specific and event-free survival analysis (n = 153).
Discussion
The prognostic importance of the anatomic location of osteosarcoma within a long bone (proximal or distal metaphysis) has been questioned [Citation5,Citation6], particularly in regard to other confounding factors, such as larger tumor size or resistance to chemotherapy [Citation7,Citation8]. However, similar to other prior studies [Citation1,Citation3,Citation4,Citation11], proximal tumor site had significant negative prognostic implications in our cohort of patients with extremity long bone osteosarcoma.
It is unknown why proximal tumors have poor prognosis compared to distal tumors. The most straightforward hypothesis is that the incidence and growth potential of osteosarcoma is directly proportional to the activity of the closest adjacent physis. Much accumulated data on osteosarcoma shows that their incidence at specific anatomic sites is proportional to the percentile growth of the adjacent physis [Citation10]. For example, the proximal humeral physis accounts for 80% of the longitudinal growth of this bone, and most osteosarcomas of the humerus involve the proximal metaphysis. The same explanation could therefore be applied regarding the prognosis of osteosarcoma – that prognosis is also related to the activity of the adjacent physis. However, in contrast to the humerus and tibia, the incidence of femoral osteosarcoma is far greater in its distal aspect, where prognosis is better. Other possibilities are variations in regional blood flow to or the timing of physeal plate closure in the proximal and distal epiphyses of extremity long bones, but there is little supportive evidence for these hypotheses.
Although there could be biological factors that determine more aggressive clinical courses of tumors arising in proximal long bones, a more likely factor accounting for this difference is that surgical resection of proximal osteosarcoma presents greater technical challenges than in the distal long bones. However, there were no significant differences in resection margin status between proximal and distal tumors in our cohort and resection margin status was included in the multivariate proportional hazard regression model as a confounding variable. Therefore, until technical differences in the difficulty of resection between various anatomic sites can be modeled better, the potential effect of variation in tumor biology on clinical outcomes will be difficult to determine.
In summary, our results demonstrate that proximal location of osteosarcoma in the long bone of an extremity portends a worse clinical outcome than tumors located in the distal long bones and is an important, independent, negative prognostic factor for disease-specific survival even after controlling for other well accepted prognostic factors.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2002;20:776–90.
- Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, et al. Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 2008;97:456–61.
- Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol 1997;15:76–84.
- Whelan JS, Jinks RC, McTiernan A, Sydes MR, Hook JM, Trani L, et al. Survival from high-grade localised extremity osteosarcoma: combined results and prognostic factors from three European Osteosarcoma Intergroup randomised controlled trials. Ann Oncol 2012;23:1607–16.
- Iwata S, Nakamura T, Gaston CL, Carter SR, Tillman RM, Abudu A, et al. Diaphyseal osteosarcomas have distinct clinical features from metaphyseal osteosarcomas. Eur J Surg Oncol 2014;40:1095–100.
- Jeon DG, Song WS, Cho WH, Kong CB, Cho SH. Proximal tumor location and fluid-fluid levels on MRI predict resistance to chemotherapy in Stage IIB osteosarcoma. Clin Orthop Relat Res 2014;472:1911–20.
- Cho WH, Song WS, Jeon DG, Kong CB, Kim MS, Lee JA, et al. Differential presentations, clinical courses, and survivals of osteosarcomas of the proximal humerus over other extremity locations. Ann Surg Oncol 2010;17:702–8.
- Picci P, Bacci G, Campanacci M, Gasparini M, Pilotti S, Cerasoli S, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer 1985;56:1515–21.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. 648 p.
- Czerniak B. Osteosarcoma. Dorfman and Czerniak's Bone Tumors. 2nd ed. Philadelphia, PA: Elsevier; 2016.
- Kim MS, Lee SY, Cho WH, Song WS, Koh JS, Lee JA, et al. An examination of the efficacy of the 8 cm maximal tumor diameter cutoff for the subdivision of AJCC Stage II osteosarcoma patients. J Surg Oncol 2008;98:427–31.
