Abstract
Purpose: We studied anterior cruciate ligament (ACL) tibial insertion architecture in humans and investigated regional differences that could suggest unequal force transmission from ligament to bone. Materials and methods: ACL tibial insertions were processed histologically. With Photoshop software, digital images taken from the histological slides were collaged, contour lines were drawn, and different gray values were filled based on the structure. The data were exported to Amira software for three-dimensional reconstruction. Results: The uncalcified fibrocartilage (UF) layer was divided into three regions: lateral, medial and posterior according to the architecture. The UF zone was significantly thicker laterally than medially or posteriorly (p < 0.05). Similarly, the calcified fibrocartilage (CF) thickness was significantly greater in the lateral part of the enthesis compared to the medial and posterior parts (p < 0.05). Conclusions: The UF quantity (more UF laterally) corresponding to the CF quantity (more CF laterally) at the ACL tibial insertion provides further evidence suggesting that the load transferred from the ACL to the tibia was greater laterally than medially and posteriorly.
Introduction
The anterior cruciate ligament (ACL) inserts into the subchondral bone via a multi-tissue interface, which enables the soft and hard tissues to function in unison and in turn facilitates physiological loading and joint motion. An ACL tibial insertion consists of four distinct tissue layers of transition, namely ligaments, uncalcified fibrocartilage (UF), calcified fibrocartilage (CF), and bone (Citation1). The region-dependent matrix organization and the interface subdivision into uncalcified and calcified regions causes a gradual increase in mechanical properties across the interface regions and minimizes stress levels, enabling effective load transfer from ligament to bone (Citation2). The greater compression or shear level, the more prominent the UF (Citation1,Citation3,Citation4). The CF zone thickness and interface extent may be related to the physiological strength and loading of the tendon or ligament (Citation5). Understanding the regional variations in the ACL tibial insertion fibrocartilage would help to explore the ACL’s mechanical property.
Previous studies about kinematics and mechanical property are based on ACL differentiation into functional bundles (Citation6,Citation7). It is widely accepted that the ACL is composed of two functional bundles; the anteromedial (AM) bundle and the posterolateral (PL) bundle (Citation8–11). However, the human ACL cannot be clearly and completely divided into two separate parts, as determined in the study (Citation12). To circumvent the experimental challenges associated with separating ACL into different functional bundles by conventional methods, we studied the regional UF and CF spatial architecture and thickness at the ACL-to-bone interface.
We proposed a hypothesis in which there were regional variations in the ACL tibial insertion fibrocartilage. To explore the different force transmission patterns from the ACL to the tibia, our study analyzed the regional variations in the UF and CF of the ACL tibia enthesis. By studying the regional UF and CF spatial architecture and thickness, we indirectly divided ACL into different functional bundles with histological evidence.
Materials and methods
Experimental specimens preparation
Fifteen human knee joints from 15 healthy humans (9 male and 6 female, aged 23–48 y) were provided by our Hospital’s Bone Tissue Engineering Center. This study complied with the Helsinki Declaration and was approved by our Hospital’s Ethics Committee. The subjects were killed in traffic accidents and were judged as having no signs of gross bony deformity, previous fractures or degenerative diseases in the knee by X-ray and magnetic resonance imaging (MRI). ACL tibial insertions were removed from 15 fresh knee joints within 48 h of death. The patellar tendon was removed after the retraction of skin and s.c. fascia, and an incision was made into the joint capsule to expose the femoral condyle and tibial plateau. The tibial insertion was then identified and excised. The samples contained the distal part of the ligament (4 mm) and its insertion together with the bone (4 mm). All the ACLs were not divided into the AM and PL bundles macroscopically. Each block was fixed in 10% neutral buffered formalin, decalcified with 5% nitric acid, dehydrated through a graded alcohol series, cleared in xylene, and embedded in paraffin wax. Serial 5-μm thick transverse sections were cut and mounted on glass slides at 50-μm intervals.
Two-dimensional imaging
The sections were stained with hematoxylin for 3 min and differentiated in 1% acid–alcohol for 15 s, followed by staining in 0.02% aqueous fast green for 3 min and being counterstained with 0.1% Safranin O for 3 min, as described previously (Citation13). Finally, the sections were dehydrated in an ethanol serial dilution, cleared in xylene, and mounted onto glass slides using neutral gum. Each stained section was observed under light microscope and photographed using a photomicroscope equipped with a CCD video camera. With Photoshop 8.0 software (Adobe Company, San Jose, CA), serial digital images taken from each transverse histological slide were automatically collaged to obtain the total image. The four zones, fibrous tissue, UF, CF, and bone, were distinguished according to their histomorphology and the staining, as described previously (Citation14), and the contour lines drawn manually. The fibrous tissue region was characterized by the presence of spindle-shaped fibroblasts lying between the collagen fibers and the region was stained green. The UF range was estimated from the tidemark to the furthest recognizable chondrocyte within the ligament, and the area was defined as the red-stained area resulting from Safranin O staining. The CF area was defined as the area between the tidemark and the subchondral bone. The large rounded fibrocartilage cells were conspicuous in the CF zone which was stained red. The bone zone had organized lamellar bones contrasting with the irregular adjacent CF area contours. The bone area was stained blue or green. The two-dimensional images were filled with different gray values manually using the Photoshop 8.0 software.
Three-dimensional reconstruction and data analysis
We fed the total transverse gray images into Amira 5.2.0 software (VSG Company, Richmond, VIC, Australia) after processing with Photoshop 8.0 software. We reconstructed the 3D model using the Amira software, in which blue represented fibrous tissue, yellow denoted UF, red denoted CF and green suggested bone. Combined with Amira 5.2.0 software, insertion architecture was observed clearly. We divided the UF layer into several regions according to the architecture. In the proximal-distal direction, three-dimensional UF graphics were projected onto a plane, where the average thickness of each region was measured using Amira 5.2.0 software. Based on the UF divided regions, the CF thickness was also measured. The mean thickness and standard deviation of the two layers of 15 samples were calculated in each divided region.
Statistical analysis
The UF thickness was compared by one-way analysis of variance (ANOVA). The CF thickness was compared by ANOVA. A One-Way ANOVA (Analysis of Variance) was a statistical technique by which we could test if three or more means were equal. It tested if the value of a single variable differed significantly among three or more levels of a factor. The p value for significance was set at 0.05.
Results
Histology
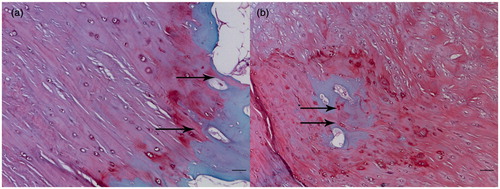
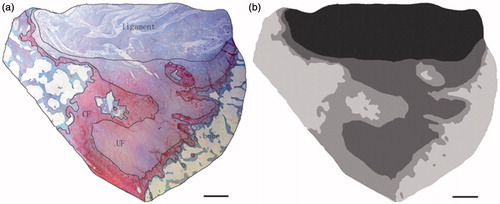
The tissue transition in the insertion showed regional differences leading to several tissues appearing simultaneously in one transverse section (). The ligament region was characterized by the presence of elongate fibroblasts lying between collagen fibers, and the region was stained green (). The UF region was stained red and the fibrocartilage cells were more rounded and lay in lacunae, surrounded by a small amount of amorphous extracellular matrix (). The tidemark was a basophilic line separating the UF and CF and represented a calcification front that was relatively straight. The ligament’s collagen fibers continued across the tidemark, as they passed from UF to CF. The junction between the CF and subchondral bone was highly irregular in contrast to the junction between UF and CF (). The distinction between four layers and image processing were mentioned in the methods ().
Figure 1. Serial transverse sections show several tissues appearing simultaneously in each section. Note the fibrocartilage appearing red and ligament appearing green (A, anterior; P, posterior; M, medial; L, lateral). Scale bar = 1 mm. Safranin O/fast green staining.
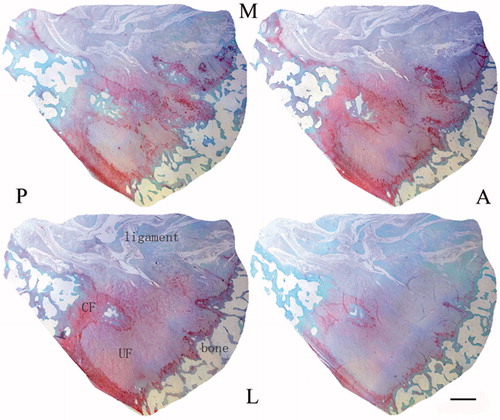
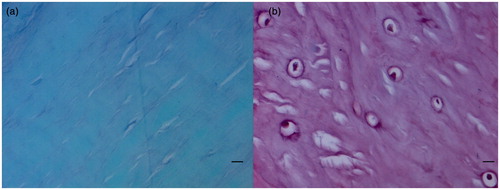
Figure 2. Histological sections of the ACL tibial insertions. Scale bars = 0.01 mm. Safranin O/fast green staining. (a) There are fusiform and spindle-shape fibroblasts and a high density of collagen bundles in ligaments stained green. (b) The fibrocartilage cells are round and ovoid in the UF region, and the area is stained red.
Three-dimensional reconstruction model and data analysis
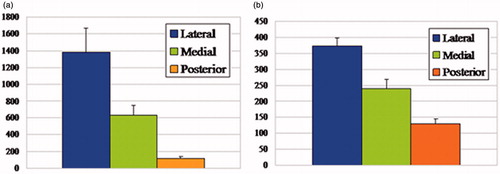
The reconstructed models demonstrated the characteristic spatial structure of fibrocartilage of ACL tibial insertion. We observed the layers’ spatial structures at different views in a 360 degree arc ( and ). According to the architecture, the UF layer was divided into 3 regions: the lateral, medial, and posterior (). We found the lateral part of the UF layer appeared superficially compared with the medial part. The transition of tissues from ligament to UF in the insertion first occurred in the lateral part, and there was almost no intersection of the lateral and medial UF parts. The architecture varied with the lateral and medial regions appearing as separate plateaus. Compared with the lateral and medial parts, the posterior UF part demonstrated different spatial structure and presented a hollow slope shape. It showed the significantly different structure units and suggested different functional units. The CF shape was extremely irregular, and there was a jigsaw-like interlocking of CF pieces and bone in three regions. The UF zone was significantly thicker laterally (1386 µm ± 283 µm) than medially (629 µm ± 117 µm) or posteriorly (115 µm ± 21 µm) (p < 0.05) (). A significant difference was observed between the medial and posterior parts. The UF thickness was significantly higher medially (p < 0.05). The CF layer thickness was significantly greater in the lateral part (373 µm ± 76 µm) of the enthesis than the medial (241 µm ± 56 µm) and posterior parts (128 µm ± 25 µm) (p < 0.05) (). A significant difference was also observed between the medial and posterior parts. The CF thickness was significantly higher medially (p < 0.05). The methods and results were reliable and reproducible.
Figure 5. The three-dimensional model of ACL tibial insertions. Blue represented fibrous tissue, yellow denoted UF, red standed for CF, and green denoted bone. The junction between the CF and subchondral bone was highly irregular, with a jigsaw-like interlocking of CF pieces and bone promoting a strong union. Scale bar = 2 mm.
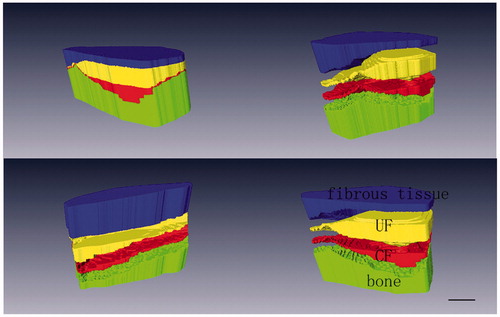
Figure 6. The exhibition of UF and CF architecture. The models allowed different views in a 360 degree arc. Scale bar = 2 mm. (a) Schematic drawings of the model perspective. (b) The spatial structure of the UF. (c) The spatial structure of the CF. (d) The spatial structure of the UF and CF complex.
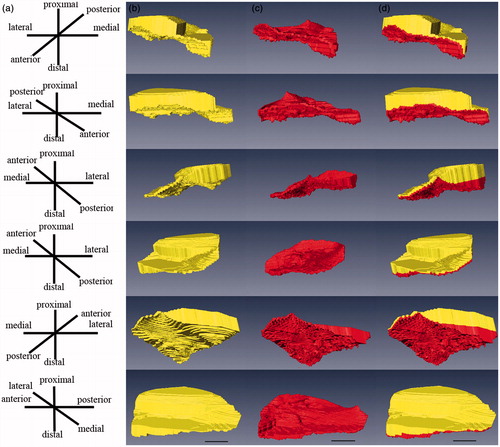
Figure 7. Schematic drawings of the UF layer divided into three regions. The lateral part of UF layer appeared superficially compared to the medial part. The transition of tissues from ligament to UF in the insertion first occured in the lateral part, and there was almost no intersection of the lateral and medial UF parts. The architecture varied with the lateral and medial regions appearing as separate plateaus. Compared with the lateral and medial parts, the posterior UF part demonstrated different spatial structures and presented a hollow slope shape (P, posterior; M, medial; L, lateral). Scale bar = 2 mm.
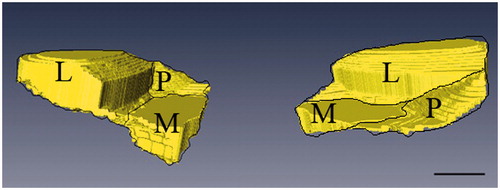
Figure 8. (a) The UF thickness in the lateral, medial, and posterior parts of the ACL tibial insertion (mean values ± SD, micrometers), with significant differences (p < 0.05) among the three regions. The lateral UF zone was significantly thicker compared with medial and posterior parts. (b) The CF thickness in the lateral, medial, and posterior parts of the ACL tibial insertion (mean values ± SD, micrometers), with significant differences (p < 0.05) among the three regions. The CF zone was significantly thicker laterally compared with medial and posterior parts.
Discussion
Tendons and ligaments detect changes in mechanical loads and alter the fibrocartilaginous matrix composition at sites under mechanical stress (Citation3). Therefore, analyzing enthesis architecture provides valuable information related to stress patterns transmitted to the joint. Toumi et al. (Citation14) investigated the regional differences in the quadriceps enthesis and showed that a greater amount of total calcified and uncalcified tissues at the quadriceps tendon enthesis occurred centrally and laterally than medially. The findings suggested a higher lateral and central mechanical stress at the proximal quadriceps tendon enthesis, inducing a lateral patellar translation, which was potentially a risk factor for knee osteoarthritis. To explore the different force transmission patterns from ACL to the tibia, we analyzed the regional variations in the UF and CF zone of the ACL tibia enthesis.
The results showed a regional variation in the UF and CF at the ACL tibia insertion. The data showed that a greater amount of total UF and CF tissue at the ACL tibia insertion occurred laterally than medially and posteriorly. Fibrocartilage is found in areas of the body subject to high mechanical loads (Citation15). The presence of fibrocartilage between the ligament and bone suggests a need for gradual change in mechanical properties between hard and soft tissues. This would dissipate stress concentration at the bony interface by promoting a gradual bending of collagen fibers (Citation1). The UF quantity (more UF laterally than medially and posteriorly) corresponding to the CF quantity (more CF laterally than medially and posteriorly) at the ACL tibial insertion provided further evidence suggesting that the load transferred from the ACL to the tibia was greater laterally than medially and posteriorly.
The UF acts like a rubber grommet on an electrical plug, protecting the tendon or ligament from compression (Citation3), and enables the highly concentrated forces in the tendon or ligament to be redistributed over a larger area. The finite element analyses from Giori et al. (Citation16) showed that regions where UF was present in the rabbit flexor digitorum profundus tendon correlate well with high compressive stress areas. The greater the compression or shear level, the more prominent the UF (Citation1,Citation4). Our results suggested increased UF laterally at the ACL tibia enthesis rather than in the medial and posterior parts. It was plausible that during the knee movement, ACL was more compressed laterally than medially and posteriorly.
A good correlation was also observed between the UF quantity at an enthesis and the degree of movement between tendon and bone (insertional angle) (Citation3). Insertional angles were located between the long axis of the ligament or tendon and the bone. The angle changed during the joint movement. The enthesis of the flexor carpi ulnaris tendon attached to the pisiform shows the greatest UF area. The UF in the pisohamate and pisometacarpal ligaments attached to the pisiform is greater at the enthesis. In the pisohamate and pisometacarpal ligaments, the proximal enthesis UF is more extensive than the distal. It is related to the more conspicuous change in insertional angles of the flexor carpi ulnaris tendon and pisiform than the two ligaments during wrist movements (Citation17). The regional variations in the UF quantity at different entheses suggest that movement is the mechanical stimulus that triggers the metaplasia of fibroblasts to fibrocartilage cells. Our results demonstrated that the UF zone was thicker laterally than medially and posteriorly and suggested the insertional angle of ACL lateral fibers was more conspicuous than medial and posterior fibers during knee movements.
Gao and Messner (Citation18) have suggested the CF-bone interface shape and surface area at ligament insertions is determined by the tensile loads to which a ligament is subjected around puberty, but its subchondral plate thickness may respond to loads beyond puberty and reflect motion at the hard soft tissue interface. This finding is consistent with the earlier suggestions of Evans et al. (Citation19), that differences in the subchondral plate thickness at entheses relate to differences in their tensile loading. The CF zone thickness and the extent of the interface that it provides for the bone may be related to the physiological strength and loading of the tendon or ligament (Citation5). We suggested that a higher physiological strength for ACL lateral fibers compared with the medial and posterior fibers generated more mechanical stress, which in turn translated into differential CF thickness in the insertion.
This was the first demonstration of histological 3D architecture of ACL tibial insertion, which provided more information compared with the 2D image. We divided ACL into 3 different functional bundles, including lateral, medial and posterior, based on the UF regions, and measured the average UF and CF thickness using Amira 5.2.0 software. UF and CF thickness differences in each part suggested that the ACL lateral bundle was more compressed, with a more conspicuous insertional angle and higher physiological strength, than medial and posterior bundles during knee movement. In this study, we indirectly divided ACL into different functional bundles based on histological evidence, in addition to classification based on macroscopic anatomy or arthroscopy. The ACL lateral bundle was subjected to high mechanical loads.
The ACL function is closely related to the anatomical structure and the precise anatomy of the ACL remains controversial. It is widely accepted that the ACL is composed of two functional bundles, the AM and the PL bundles (Citation8–11). They can be distinguished by the various tension patterns during knee range of motion and also with MRI along oblique sagittal and oblique coronal planes (Citation20,Citation21). However, conflicting evidence about the anatomic bundle division remains. Amis and Dawkins (Citation22) identified three bundles during cadaveric knee examinations. In a histological study, Odensten and Gillquist (Citation12) found no evidence of distinct bundles in the ACL. The distinction between the 2 bundles of the fetal ACL was observed clearly in the transverse and sagittal histologic sections, with a well-defined septum of vascularized connective tissue dividing the AM and PL bundles (Citation23). However, we found no conspicuous septum dividing the AM and PL bundles in the transverse histologic sections. Evidence was inadequate to indicate that the lateral and medial bundles in our results were equivalent to the PL and AM bundles mentioned in previous studies. The relationship between the two requires further investigation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This work is supported by grants from the National Natural Science Foundation of China (81000806).
References
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat 1986;149:89–100
- Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, Hung CT, Lu HH. Characterization of the structure-function relationship at the ligamentto-bone interface. Proc Natl Acad Sci USA 2008;105:7947–52
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments–an adaptation to compressive load. J Anat 1998;193:481–94
- Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons-tendon “entheses”. Comp Biochem Physiol A Mol Integr Physiol 2002;133:931–45
- Fredericson M, Yoon K. Physical examination and patellofemoral pain syndrome. Am J Phys Med Rehabil 2006;85:234–43
- Bedi A, Musahl V, O'Loughlin P, Maak T, Citak M, Dixon P, Pearle AD. A comparison of the effect of central anatomical single-bundle anterior cruciate ligament reconstruction and double-bundle anterior cruciate ligament reconstruction on pivot-shift kinematics. Am J Sports Med 2010;38:1788–94
- Yasuda K, van Eck CF, Hoshino Y, Fu FH, Tashman S. Anatomic single- and double-bundle anterior cruciate ligament reconstruction, part 1: basic science. Am J Sports Med 2011;39:1789–99
- Harner CD, Baek GH, Vogrin TM, Carlin GJ, Kashiwaguchi S, Woo SL. Quantitative analysis of human cruciate ligament insertions. Arthroscopy 1999;15:741–9
- Chhabra A, Starman JS, Ferretti M, Vidal AF, Zantop T, Fu FH. Anatomic, radiographic, biomechanical, and kinematic evaluation of the anterior cruciate ligament and its two functional bundles. J Bone Joint Surg Am 2006;88:2–10
- Petersen W, Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res 2007;454:35–47
- Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med 2007;35:223–7
- Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg Am 1985;67:257–62
- Zhang Y, Wang F, Tan H, Chen G, Guo L, Yang L. Analysis of the Mineral Composition of the Human Calcified Cartilage Zone. Int J Med Sci 2012;9:353–60
- Toumi H, Larguech G, Filaire E, Pinti A, Lespessailles E. Regional variations in human patellar trabecular architecture and the structure of the quadriceps enthesis: a cadaveric study. J Anat 2012;220:632–7
- Benjamin M, Tyers RN, Ralphs JR. Age-related changes in tendon fibrocartilage. J Anat 1991;179:127–36
- Giori NJ, Beaupré GS, Carter DR. Cellular shape and pressure may mediate mechanical control of tissue composition in tendons. J Orthop Res 1993;11:581–91
- Adamczyk C, Milz S, Tischer T, Putz R, Benjamin M. An immunohistochemical study of the extracellular matrix of entheses associated with the human pisiform bone. J Anat 2008;212:645–53
- Gao J, Messner K. Quantitative comparison of soft tissuebone interface at chondral ligament insertions in the rabbit knee joint. J Anat 1996;188:367–73
- Evans EJ, Benjamin M, Pemberton DJ. Variations in the amount of calcified tissue at the attachments of the quadriceps tendon and patellar ligament in man. J Anat 1991;174:145–51
- Steckel H, Vadala G, Davis D, Fu FH. 2D and 3D 3-tesla magnetic resonance imaging of the double bundle structure in anterior cruciate ligament anatomy. Knee Surg Sports Traumatol Arthrosc 2006;14:1151–8
- Steckel H, Vadala G, Davis D, Musahl V, Fu FH. 3-T MR imaging of partial ACL tears: a cadaver study. Knee Surg Sports Traumatol Arthrosc 2007;15:1066–71
- Amis AA, Dawkins GP. Functional anatomy of the anterior cruciate ligament: fibre bundle actions related to ligament replacements and injuries. J Bone Joint Surg Br 1991;73:260–7
- Ferretti M, Levicoff EA, Macpherson TA, Moreland MS, Cohen M, Fu FH. The fetal anterior cruciate ligament: an anatomic and histologic study. Arthroscopy 2007;23:278–83