Abstract
Deposition of amyloid, derived from the polypeptide hormone islet amyloid polypeptide (IAPP; ‘amylin’) is the single most typical islet alteration in type 2 diabetes. Islet amyloid was described as hyalinization already in 1901, but not until 1986 was it understood that it is a polymerization product of a novel β-cell regulatory product. The subject of this focused review deals with the pathogenesis and importance of the islet amyloid itself, not with the biological effect of the polypeptide. Similar to the situation in Alzheimer's disease, it has been argued that the amyloid may not be of importance since there is no strict correlation between the degree of islet amyloid infiltration and the disease. However, it is hardly discussable that the amyloid is important in subjects where islets have been destroyed by pronounced islet amyloid deposits. Even when there is less islet amyloid the deposits are widely spread, and β-cells show ultrastructural signs of cell membrane destruction. It is suggested that type 2 diabetes is heterogeneous and that in one major subtype aggregation of IAPP into amyloid fibrils is determining the progressive loss of β-cells. Interestingly, development of islet amyloid may be an important event in the loss of β-cell function after islet transplantation into type 1 diabetic subjects.
Introduction
It is now 25 years since we elucidated the nature of the amyloid in the islets of Langerhans (Citation1). Deposition of amyloid is the most characteristic alteration in the islets of Langerhans in type 2 diabetes and was described already 110 years ago (Citation2,Citation3); it was for long time named hyalinization of the islets. A resemblance to amyloid was noted at an early date, and when the nature of this substance was debated the name ‘para-amyloid’ was sometimes used for deposits like those in the islets (Citation4). However, not until the studies by Ehrlich and Ratner (Citation5) was the material accepted as a ‘real’ form of amyloid. It may be mentioned that the discussion about inclusion criteria for amyloid is still on-going (Citation6). For a long time, the interest for this alteration, which is quite characteristic for type 2 diabetes, was generally low, but it was an enigma mainly for pathologists who noticed the islet alteration when examining autopsy specimens. The low interest in amyloid among researchers in the diabetes field might have been due to the fact that islet amyloid is missing in mouse and rat models of diabetes. The reason for this absence became obvious soon after our description of the nature of human (and feline) islet amyloid (Citation1,Citation7). Another reason for the lack of interest is that islet amyloid is not solely seen in association with diabetes; it occurs also in non-diabetic subjects but less commonly and to a lower degree (Citation8–10). The finding that islet amyloid is composed by a previously unknown polypeptide hormone immediately increased the interest in islet amyloid.
The elucidation of the islet amyloid nature
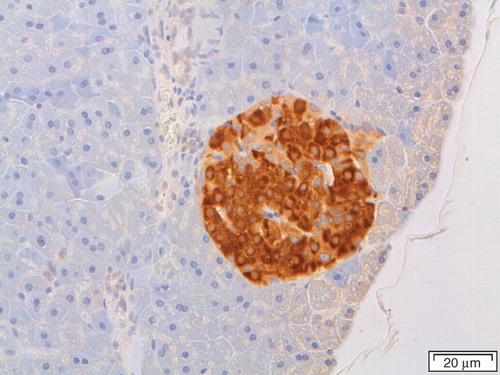
Gellerstedt, a professor of pathology at Uppsala University, noted already in 1938 that the Congo red staining property of islet amyloid was slightly different from that of systemic amyloid (Citation4). Also histochemical studies indicated that the islet amyloid was different (Citation11,Citation12). However, only an amino acid sequence analysis could determine the nature of the material. It was not an easy task to extract, purify, and determine the nature of the main protein in islet amyloid. In 1971 the two first fibril proteins from systemic amyloidosis had been purified and determined by Edman degradation. One was found to be of immunoglobulin light chain origin (Citation13), while the second turned out to be a previously unknown protein, now called AA (Citation14). The third amyloid fibril protein to be characterized was calcitonin (or procalcitonin) in the C-cell tumor thyroid medullary carcinoma (Citation15). In all these instances, the tissue material serving for protein purification was very rich in amyloid, making identification of the main protein comparatively easy. Dealing with amyloid in the islets of Langerhans was a completely different problem. This amyloid is strictly limited to the around 1 million islets which constitute about 1% of the total pancreatic mass. A second problem was the unusual insolubility of this amyloid (Citation12). Previously studied amyloid proteins had been purified after solubilization in guanidine hydrochloride, but islet amyloid turned out to be completely insoluble in this solvent. After years of trials, the solution to the problem came with a β-cell tumor (insulinoma), rich in amyloid. With this material a new approach was used, including solubilization in concentrated formic acid, used for identification of the Aβ protein (Citation16), a new purification method with the aid of high-performance liquid chromatography and access to a new sensitive gas phase sequenator. In this way, the nature of the protein was finally elucidated (Citation1). Surprisingly, it turned out to be a novel β-cell protein, not related to insulin or its precursor. It was initially named insulinoma (or islet) amyloid peptide (IAP), which soon changed to islet amyloid polypeptide (IAPP) since the abbreviation IAP was already used. Further analyses, also of protein purified from amyloid derived from human and cat islets (), revealed a 37-amino acid residue polypeptide belonging to the calcitonin gene-related peptide (CGRP) family (Citation7,Citation17). Soon afterwards, our findings were verified by another research group (Citation18). Parts of this group later named the peptide ‘amylin’ (Citation19). By immunohistochemistry (Citation7,Citation20), immune electron microscopy (Citation21,Citation22), and later in-situ hybridization (Citation23) it was shown that IAPP is a product of islet β-cells (Citation24). IAPP is stored together with insulin in the secretory vesicles. It is located to the halo region where also proinsulin and C-peptide are located. Although most of the circulating IAPP is derived from islet β-cells, there is IAPP expression in some gastrointestinal endocrine cells, in certain peripheral ganglia, and in the brain (for review, see (Citation25)).
Figure 1. Pancreatic section of case IsN13 with islets filled with amyloid, stained with Congo red. This material was used to purify the IAPP giving the first amino acid sequence from human islet origin. There are signs of pronounced autolysis, which did not affect the quality of the purified peptide. There was also partial exocrine atrophy which was helpful in that it made the amyloid more concentrated.
IAPP in animal species
IAPP is a conserved molecule and is expressed in mammals (Citation26,Citation27) (), birds (Citation28), fishes (Citation29,Citation30), and reptiles (Westermark and Westermark, unpublished) although islet amyloid is seen only in a limited number of species. As mentioned in the introduction, islet amyloid does not appear in mouse or rat islets. It is, however, a typical alteration in diabetes in many non-human primate species (Citation31,Citation32) as well as in cat species (Citation33–35). Structural studies of islet amyloid in several mammalian species revealed that IAPP is a strongly conserved molecule but that there are considerable species variations in a middle segment of the peptide. It turned out that mouse and rat IAPP, which are identical, carry three proline residues in the 20–29 segment, where the human molecule has none and the cat has one (Citation26,Citation36). While a synthetic peptide corresponding to human IAPP 20–29 is extremely fibrillogenic in vitro, that corresponding to the rat/mouse molecule is not (Citation37). Consequently, it turned out that the reason why islet amyloid is not found in these rodents is simply that their IAPP is not amyloidogenic. Parenthetically, it may be mentioned that these species discrepancies have been utilized by a pharmaceutical company to make a non-fibrillogenic IAPP variant, used for treatment of type 1 and type 2 diabetes.
Amyloid in the degu: an interesting exception
There is a remarkable exception from the rule that islet amyloid does not occur in species with apparently non-fibrillogenic IAPP, and that is the South American hystricomorphous rodent degu (Octodon degus). This animal is prone to develop diabetes in captivity (Citation38). Degu IAPP has proline residues at positions 28 and 29 (Citation39). In spite of this, islet amyloid is common in elderly and diabetic animals (). Direct amino acid sequence analysis of purified degu islet amyloid revealed surprisingly that the fibril protein in this species is derived from insulin (Citation40). In a way, this finding closed the circle, since insulin was, for natural reasons, believed to be the fibril protein in human islet amyloid (Citation41) before IAPP was discovered. Insulin is an amyloid protein also in human, although an iatrogenic one; amyloid ‘tumors’ may appear at the site of repeated insulin injections in type 1 diabetic subjects (Citation42,Citation43).
Morphology of islet amyloid
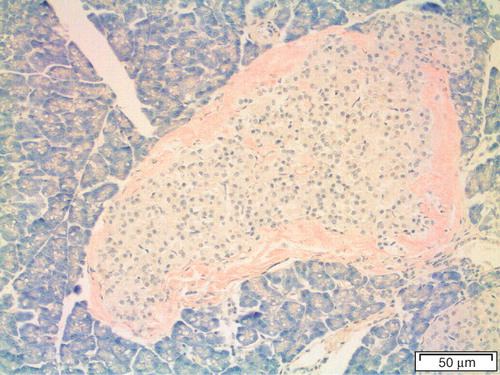
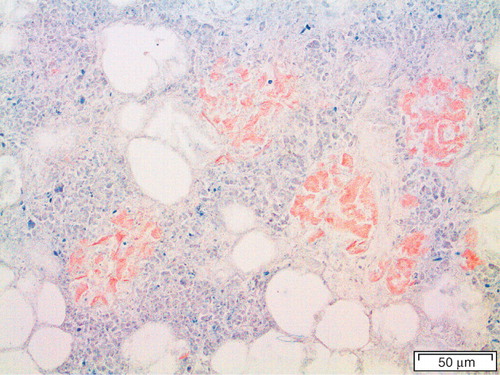
Islet amyloid is restricted to the islet area and is not found systemically or in other parts of the pancreas. In human, islet amyloid is extracellular, and it has been difficult to find certain intracellular amyloid. When the deposits are small, amyloid can be seen as thin layers between endocrine cells and capillaries and are tapestrying the outside of the islet. When larger, the deposits can form large masses completely remodeling the islets (). Amyloid can be visualized with ordinary amyloid dyes such as Congo red and shows the typical birefringence that can vary between yellow and green (), although erroneously often referred to as ‘apple green’ (Citation44). It should be noted that the affinity of this kind of amyloid for Congo red often is weak or very weak, making it easy to underestimate the amount of deposited material or even to totally miss the amyloid. By definition, islet amyloid consists of thin fibrils, around 10 nm in width, but with an undetermined length. Within the deposits, the fibrils are not organized, but close to β-cells there is often an orientation of bundles of fibrils towards the cell membrane (Citation24). Typically, bundles of fibrils run into deep pockets of β-cells, seemingly penetrating the cell membrane (). A conspicuous feature of β-cells close to islet amyloid is the distortion of the architecture of the outer part of the cells and loss of an evident basement membrane (Citation24). The lack of intracellular amyloid in β-cells of diabetic individuals does not necessarily mean that aggregation of IAPP to fibrils may not take place here. In models with more rapidly developing islet amyloid, e.g. in human islets transplanted into nude mice or in islets cultured in vitro, small amyloid deposits develop within a few days or weeks (Citation45,Citation46). This very first amyloid is intracellular. We have suggested that this first amyloid leads to apoptosis of the cell, leaving the amyloid, which is resistant to degradation, extracellularly where it seeds further amyloid formation from exocytosed IAPP (Citation46). Such an event might explain the deep pockets into β-cells formed by the fibrils when these are elongated by addition of new IAPP molecules at the cellular side (Citation47).
Figure 4. Human islet from a diabetic subject. Most of the islet has been converted into amyloid, but there are still cords of cells, a majority being β-cells, most probably dysfunctional. Congo red. Bar: 50 μm.
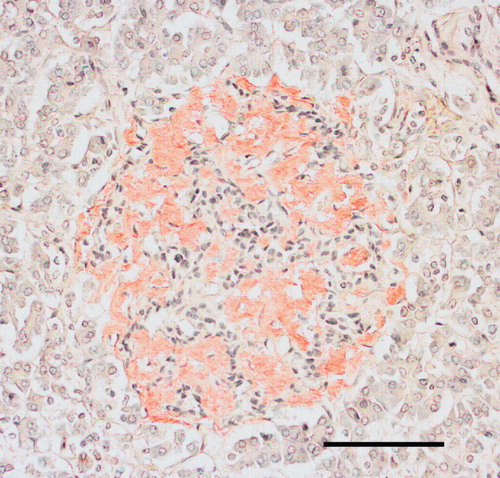
Figure 5. Section from the pancreas of a diabetic subject with severe islet infiltration of amyloid in all islets, visualized with Congo red. The section in A is seen in ordinary light, while in B polarized light with crossed polars has been used. A bright yellow birefringence is evident.
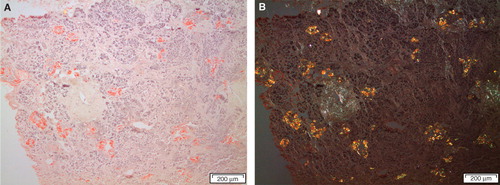
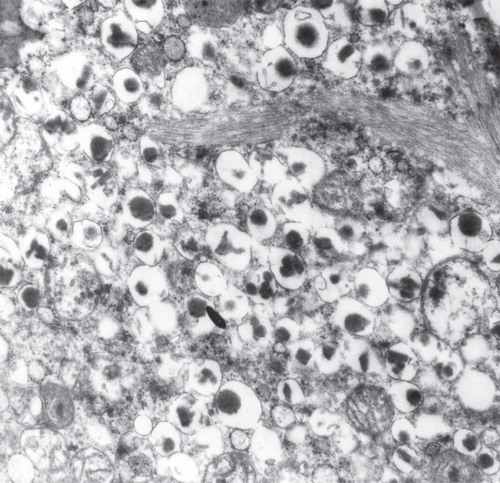
Figure 6. Electron micrograph showing a part of a β-cell from a diabetic subject. There are bundles of typical amyloid fibrils which penetrate deeply into the cells. Note that the cell is filled with characteristic insulin granules.
The spreading of islet amyloid
An interesting phenomenon is that even very small amyloid deposits are widely spread throughout an individual islet (Citation10), although other islets in the neighbourhood are free. This might indicate that the abnormality has affected all cells in the islet. Amyloidogenesis is a nucleation-dependent phenomenon and does not start until a nucleus has formed (Citation48). However, when the first fibrils have assembled, the process moves on rapidly as long as the peptide is present above a critical concentration. The widely spread amyloid in single islets may simply reflect such events. Whether or not islets may communicate so that amyloid can spread from one islet to another is still an open question.
Why does IAPP form amyloid in type 2 diabetes?
The pathogenesis of any localized form of amyloid is not clear. Although most human proteins contain segments that in theory are able to assemble into amyloid fibrils, most such parts are hidden in the molecules and may be exposed only after cleavage (Citation49). In the case of IAPP, aberrant cleavage is not necessary for fibrillogenesis since only full-length molecules have been identified in the deposits, although small amounts of propeptides can be demonstrated by immunological techniques (Citation50,Citation51). Still, aberration in the cleavage of pro-IAPP as an initial step in amyloidogenesis cannot be ruled out (Citation52). An increased local concentration of the fibrillogenic protein may be important. Over-production of insulin, often seen in type 2 diabetes as a response to insulin resistance, is associated with increased release also of IAPP since these two β-cell products are normally regulated in parallel (Citation53). IAPP is a very fibrillogenic peptide in vitro, and its native conformation must be controlled in vivo. Consequently, a further possible factor in amyloidogenesis might be a dysregulation of chaperones, normally hindering the strongly fibrillogenic molecule IAPP from making amyloid. It is not known exactly how IAPP is stored in granules and which factors normally inhibit fibril formation, but, experimentally, insulin is a strong inhibitor (Citation54–56). Although insulin is mainly stored in crystalline form in the granule core and IAPP in the halo region, it is possible that there is enough insulin in the halo to inhibit fibril formation. Proinsulin, present in the halo, is also an inhibitor (Citation54). Finally, there may be unknown inhibitors and promoters in IAPP amyloidogenesis. Among additional components present in all forms of amyloid and which may affect amyloidogenesis are heparan sulfate (Citation57) and serum amyloid P-component (SAP) (Citation58,Citation59).
Is islet amyloid of any importance in type 2 diabetes?
This question can be rephrased as to whether aggregated IAPP in any form is pathogenically important since there is evidence that not fully developed amyloid fibrils but smaller, oligomeric assemblies exert toxic effects on β-cells and thereby kill them (Citation60). It has even been suggested that the amyloid itself is innocent and that its formation rather is a protection mechanism by which the dangerous oligomeric species are taken care of (Citation61). In this paper, I will not discuss oligomers more, and interested readers are referred to a recent review (Citation25). However, when looking at a pancreatic section from a patient with type 2 diabetes where almost all of the islets are converted to amyloid (), it is very difficult to believe that the amyloid itself is without importance. Both insulin (Citation62) and IAPP(Citation63) are normally released in pulses. It is tempting to believe that the tightly regulated pulses are disturbed by the amyloid interposed between β-cells and capillaries and most probably interfering with the cell membrane function.
Another important issue is whether formation of islet amyloid is a pathogenic event in the development of type 2 diabetes or just participating in the final destruction of islets. This question cannot be answered presently (Citation64). A reason why islet amyloid has been denied as a pathogenic mechanism in diabetogenesis is that islet amyloid does not occur in all individuals with type 2 diabetes. Interestingly, in a recent study on a baboon colony, islet amyloid appeared before the development of diabetes, and the amount of amyloid correlated well with the progression of the disease (Citation65). It has not been possible to demonstrate such a direct association in human. What we call type 2 diabetes is heterogeneous in human, and most probably several different mechanisms act in the pathogenesis. It is quite possible that, in one major subgroup of individuals, aggregation of IAPP and development of islet amyloid actually is of major importance. It is also possible that deposition of islet amyloid acts in concert with other pathogenic mechanisms. This possibility will probably not be answered until methods have been developed by which amyloid deposits can be demonstrated in vivo, e.g. by positron emission tomography (PET), or until methods to prevent islet amyloid formation or even to dissolve already formed fibrils have been introduced.
The β-cell mass in type 2 diabetes
For a long time there was no agreement concerning the question whether there is a reduction in islet volume and β-cell mass in type 2 diabetes. In early studies, types 1 and 2 diabetes were usually not clearly separated (Citation66,Citation67). Several later studies have shown a modest reduction of the islet mass in type 2 diabetes compared to age-matched controls, but there has been a considerable overlap (Citation68,Citation69). This reduction depends mainly on a smaller β-cell mass, shown in a number of studies (Citation64,Citation70–72).
In one study we measured the total pancreatic volume as well as islet volume in 12 subjects with type 2 diabetes and 15 age-matched controls (Citation69). The total islet volume in non-diabetic subjects was 1.60 ± 0.16cm3 (mean ± SEM) and in diabetic individuals 1.01 ± 0.12 cm3 (P < 0.01). In another study we found that the percentage of β-cells was significantly lower in islets of type 2 diabetic individuals compared to non-diabetic controls (43.0% ± 22% versus 59.2% ± 1.9%; P < 0.001) (Citation70). If we assume that the materials in these two studies, emerging from the same department, are comparable, an estimate of β-cell volume in type 2 diabetes and controls would be 0.43 ± 0.05 and 0.95 ± 0.09 cm3, respectively. In reality, the difference should be even greater since in this calculation the volume taken up by amyloid has not been taken into consideration. These data fit well with a finding that the β-cell area in diabetic subjects was reduced only in amyloid-containing islets (Citation64). Even more intriguing was the significant difference in β-cell percentage between islets in non-diabetic subjects with amyloid and those without any deposits (Citation70). While islets in those without any amyloid contained 64.6% ± 1.4% β-cells, the percentage of β-cells in islets in individuals with islet amyloid was 55.9% ± 2.5% (P < 0.01). This indicates that deposition of islet amyloid is directly associated with β-cell loss and not only a result of the diabetic state per se.
Limitations of islet volume determination in human
All islet volume determinations suffer from the limitation that they have had to be performed on autopsy material. That means that individuals with diabetes usually have had their disease for several years, while it would have been of greater interest to study the pancreas very early in the diabetic state, or preferably before diabetes had become established. This is presently impossible in humans until new methods have been developed. Since the pancreas is very sensitive to autolytic changes, obscuring histological details, it should be pointed out that studies have to be performed on material obtained well within 24 hours after death, something that was possible in the 1970s but is completely impossible today, at least in Sweden although it seems possible in some other countries (Citation72).
There are some good animal models which should be relevant for human type 2 diabetes. Thus, Howard showed that the diabetes which develops in Macaca nigra resembles the human form (Citation31). Islet amyloid with loss of β-cells developed before overt diabetes. A type 2-like diabetes can also be found in elderly domestic cats (Citation34,Citation73). Baboons may also offer a relevant model (see above). In addition, there are transgenic mouse and rat models over-expressing human IAPP in which diabetes associated with islet amyloid develops (for review, see (Citation25)).
Functional β-cell mass
Even in islets filled with amyloid, there are a number of β-cells filled with secretory vesicles (Citation24). These cells are in direct contact with amyloid fibrils and show morphological signs of membrane damage and are most probably not functioning in a normal way. Therefore, the functional β-cell mass may be very different from the total β-cell mass. A morphological measurement of the total β-cell mass does not give true functional mass.
Amyloid in transplanted human islets
Human islets isolated from organ donors may be transplanted to diabetic recipients, particularly in cases with diabetes which is difficult to regulate with the common regimens. Most commonly this is done via an islet infusion into the portal vein. Initially, many patients become independent of exogeneous insulin injections, but most commonly the function of the grafted islets deteriorates with time, and after a few years most individuals need insulin treatment (recently reviewed in (Citation74)). Pioneered by Andersson (Citation75), human islets can be isolated and transplanted into nude mice, some of which were made diabetic by means of alloxan injections. We found that normal human islets, transplanted under the kidney capsule (Citation45) or into the spleen or liver (Citation76), rapidly develop amyloid deposits. We therefore questioned whether amyloid may form also in clinically transplanted islets (Citation77). Recently, we had the opportunity to study the liver of one patient who had been transplanted 5 years earlier and who died from a cardiac infarction. Indeed, almost 50% of the studied islets contained IAPP amyloid, often in a substantial amount (Citation78). This finding has recently been verified by studies of additional cases (Westermark et al., unpublished). Therefore, by transplantation of normal human islets into type 1 diabetic individuals, type 2 diabetic alterations are induced in these islets.
Conclusion
Porte pointed out two findings in type 2 diabetes, providing clues to the pathogenesis: the relative hyperproinsulinemia and the deposits of amyloid in the islets (Citation79). Hyperproinsulinemia would most likely be associated with aberrant cleavage also of pro-IAPP. There is evidence that the aberrant cleavage product may start amyloid deposition by the formation of a nucleus (Citation46,Citation52). The amyloid formed may cause β-cell apoptosis and dysfunction of remaining cells. These mechanisms may also be important for the loss of function of transplanted human islets.
Acknowledgements
I have had the privilege to work with a large number of researchers over the years. A list of the names would be too long, but I would like to take the opportunity to thank them all.
Declaration of interest: Own research was supported by the Swedish Research Council, the Swedish Diabetes Association, and the Family Ernfors Fund. The author alone is responsible for the content and writing of the paper.
References
- Westermark P, Wernstedt C, Wilander E, Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986;140:827–31.
- Opie EL. On relation of chronic interstitial pancreatitis to the islands of Langerhans and to diabetes mellitus. J Exp Med. 1901;5:397–428.
- Weichselbaum A, Stangl E. Zur Kenntnis der feineren Veränderungen des Pankreas bei Diabetes mellitus. Wien Klin Wochenshr. 1901;14:968–72.
- Gellerstedt N. Die elektive, insuläre (Para-)Amyloidose der Bauchspeicheldrüse. Zugleich en Beitrag zur Kenntnis der ‘senilen Amyloidose’. Beitr Path Anat. 1938;101:1–13.
- Ehrlich JC, Ratner IM. Amyloidosis of the islets of Langerhans. A restudy of islet hyalin in diabetic and nondiabetic individuals. Am J Path. 1961;38:49–59.
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, Saraiva MJ, Amyloid fibril protein nomenclature: 2010 recommendations of the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17:101–4.
- Westermark P, Wernstedt C, Wilander E, Hayden DW, O'Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–5.
- Ahronheim JH. The nature of the hyaline material in the pancreatic islands in diabetes mellitus. Am J Path. 1943;19:873–82.
- Bell ET. Hyalinization of the islets of Langerhans in nondiabetic individuals. Am J Path. 1959;35:801–5.
- Westermark P. Quantitative studies of amyloid in the islets of Langerhans. Ups J Med Sci. 1972;77:91–4.
- Pearse AGE, Ewen SWB, Polak JM. The genesis of apudamyloid in endocrine polypeptide tumours: Histochemical distinction from immunamyloid. Virchows Arch Abt B Zellpath. 1972;10:93–107.
- Westermark P. Amyloid of human islets of Langerhans. I. Isolation and some characteristics. Acta Path Microbiol Scand C. 1975;83:439–46.
- Glenner GG, Terry W, Harada M, Isersky C, Page D. Amyloid fibril proteins: proof of homology with immunoglobulin light chains by sequence analysis. Science. 1971;172:1150–1.
- Benditt EP, Eriksen N, Hermodson MA, Ericsson LH. The major proteins of human and monkey amyloid substance: common properties including unusual N-terminal amino acid sequences. FEBS Lett. 1971;19:169–73.
- Sletten K, Westermark P, Natvig JB. Characterization of amyloid fibril proteins from medullary carcinoma of the thyroid. J Exp Med. 1976;143:993–8.
- Masters C, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–9.
- Westermark P, Wernstedt C, O'Brien TD, Hayden DW, Johnson KH. Islet amyloid in type 2 human diabetes mellitus and adult diabetic cats contains a novel putative polypeptide hormone. Am J Path. 1987;127:414–17.
- Cooper GJ, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA. 1987;84:8628–32.
- Cooper GJS, Day AJ, Willis AC, Roberts AN, Reid KBM, Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989;1014:247–58.
- Westermark P, Wilander E, Westermark GT, Johnson KH. Islet amyloid polypeptide-like immunoreactivity in the islet B cells of Type 2 (non-insulin-dependent) diabetic and nondiabetic individuals. Diabetologia. 1987;30:887–92.
- Johnson KH, O'Brien TD, Hayden DW, Jordan K, Ghobrial HKG, Mahoney WC, Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques. Am J Path. 1988;130:1–8.
- Lukinius A, Wilander E, Westermark GT, Engström U, Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989;32:240–4.
- Westermark GT, Christmanson L, Terenghi G, Permert J, Betsholtz C, Larsson J, Islet amyloid polypeptide: demonstration of mRNA in human pancreatic islets by in situ hybridization in islets with and without amyloid deposits. Diabetologia. 1993;36:323–8.
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A. 1973;359:1–18.
- Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid and diabetes mellitus. Physiol Rev. 2011. In press.
- Betsholtz C, Christmanson L, Engström U, Rorsman F, Svensson V, Johnson KH, Sequence divergence in a specific region of islet amyloid polypeptide (IAPP) explains differences in islet amyloid formation between species. FEBS Lett. 1989;251:261–4.
- Nishi M, Chan SJ, Nagamatsu S, Bell GI, Steiner DF. Conservation of the sequence of islet amyloid polypeptide in five mammals is consistent with its putative role as an islet hormone. Proc Natl Acad Sci USA. 1989;86:5738–42.
- Fan L, Westermark G, Chan SJ, Steiner DF. Altered gene structure and tissue expression on islet amyloid polypeptide in the chicken. Mol Endocrinol. 1994;8:713–21.
- Westermark GT, Falkmer S, Steiner DF, Chan SJ, Engström U, Westermark P. Islet amyloid polypeptide is expressed in the pancreatic islet parenchyma of the teleostean fish, Myoxocephalus (Cottus) scorpius. Comp Biochem Physiol B. 2002;133:119–25.
- Martínez-Álvarez RM, Volkoff H, Munoz Cueto JA, Delgado MJ. Molecular characterization of calcitonin gene-related peptide (CGRP) related peptides (CGRP, amylin, adrenomedullin and adrenomedullin-2/intermedin) in goldfish (Carassius auratus): Cloning and distribution. Peptides. 2008;29:1534–43.
- Howard CFJ. Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986;29:301–6.
- de Koning EJP, Bodkin NL, Hansen BC, Clark A. Diabetes mellitus in Macaca mulatta monkeys is characterized by islet amyloidosis and reduction in beta-cell population. Diabetologia. 1993;36:378–84.
- Johnson KH, Stevens JB. Light and electron microscopic studies of islet amyloid in diabetic cats. Diabetes. 1973;22:81–90.
- Johnson KH, Hayden DW, O'Brien TD, Westermark P. Spontaneous diabetes-islet amyloid complex in adult cats. Am J Path. 1986;125:416–19.
- Johnson KH, Wernstedt C, O'Brien TD, Westermark P. Amyloid in the pancreatic islets of the cougar (Felis concolor) is derived from islet amyloid polypeptide (IAPP). Comp Biochem Physiol. 1991;98B:115–19.
- Betsholtz C, Christmanson L, Engström U, Rorsman F, Jordan K, O'Brien TD, Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes. 1990;39:118–22.
- Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–40.
- Opazo JC, Soto-Gamboa M, Bozinovic F. Blood glucose concentration in caviomorph rodents. Comp Biochem Physiol A Mol Integr Physiol. 2004;137:57–64.
- Nishi M, Steiner DF. Cloning of complementary DNAs encoding islet amyloid polypeptide, insulin, and glucagon precursors from a new world rodent, the degu, Octodon degus. Mol Endocrinol. 1990;4:1192–8.
- Hellman U, Wernstedt C, Westermark P, O'Brien TD, Rathbun WB, Johnson KH. Amino acid sequence from degu islet amyloid-derived insulin shows unique sequence characteristics. Biochem Biophys Res Commun. 1990;169:571–7.
- Westermark P. On the nature of the amyloid in human islets of Langerhans. Histochemistry. 1974;38:27–33.
- Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB, Rennie JA, Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158–61.
- Yumlu S, Barany R, Eriksson M, Röcken C. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Path. 2009;40:1655–60.
- Howie AJ, Owen-Casey MP. Discrepancies between descriptions and illustrations of colours in Congo red-stained amyloid, and explanation of discrepant colours. Amyloid. 2010;17:109–17.
- Westermark P, Eizirik DL, Pipeleers DG, Hellerström C, Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38:543–9.
- Paulsson JF, Andersson A, Westermark P, Westermark GT. Intracellular amyloid-like deposits contain unprocessed pro islet amyloid polypeptide (proIAPP) in beta-cells of transgenic mice overexpressing human IAPP and transplanted human islets. Diabetologia. 2006;49:1237–46.
- Westermark P. Amyloid and polypeptide hormones: what is their inter-relationship? Amyloid. 1994;1:47–60.
- Jarrett JT, Lansbury PT. Seeding ‘one-dimensional crystallization’ of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73:1055–8.
- Goldschmidt L, Teng PK, Riek R, Eisenberg D. Identifying the amylome, proteins capable of forming amyloid-like fibrils. Proc Natl Acad Sci USA. 2010;107:3487–92.
- Westermark P, Engström U, Westermark GT, Johnson KH, Permerth J, Betsholtz C. Islet amyloid polypeptide (IAPP) and pro-IAPP immunoreactivity in human islets of Langerhans. Diab Res Clin Pract. 1989;7:219–26.
- Westermark GT, Steiner DF, Gebre-Medhin S, Engström U, Westermark P. Pro islet amyloid polypeptide (proIAPP) immunoreactivity in amyloid formation in the islets of Langerhans. Ups J Med Sci. 2000;105:97–106.
- Paulsson JF, Westermark GT. Aberrant processing of human proislet amyloid polypeptide results in increased amyloid production. Diabetes. 2005;54:2117–25.
- Mulder H, Ahrén B, Sundler F. Islet amyloid polypeptide and insulin gene expression are regulated in parallel by glucose in vivo in rats. Am J Physiol. 1996;271:E1008–14.
- Westermark P, Li Z-C, Westermark GT, Leckström A, Steiner DF. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 1996;379:203–6.
- Janciauskiene S, Eriksson S, Carlemalm E, Ahrén B. β cell granule peptides affect human islet amyloid polypeptide (IAPP) fibril formation in vitro. Biochem Biophys Res Commun. 1997;236:580–5.
- Jaikaran ETAS, Nilsson MR, Clark A. Pancreatic b-cell granule peptides form heteromolecular complexes which inhibit islet amyloid polypeptide fibril formation. Biochem J. 2004;377:709–16.
- Kisilevsky R, Ancsin JB, Szarek WA, Petanceska S. Heparan sulfate as a therapeutic target in amyloidogenesis: prospects and possible complications. Amyloid. 2007;14:21–32.
- Westermark P, Skinner M, Cohen AS. The P-component of amyloid of human islets of Langerhans. Scand J Immunol. 1975;4:95–7.
- Coker AR, Purvis A, Baker D, Pepys MB, Wood SP. Molecular chaperone properties of serum amyloid P component. FEBS Lett. 2000;473:199–202.
- Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–8.
- Dobson CM. Principles of protein folding, misfolding and aggregation. Semin Cell Develop Biol. 2004;15:3–16.
- Hellman B. Pulsatility of insulin release—a clinically important phenomenon. Ups J Med Sci. 2009;114:193–205.
- Juhl CB, Pørksen N, Sturis J, Hansen AP, Veldhuis JD, Pincus S, High-frequency oscillations in circulating amylin concentrations in healthy humans. Am J Physiol Endocrinol Metab. 2000;278:E484–90.
- Jurgens CA, Toukatly MN, Fligner CL, Udayasankar J, Subramanian SL, Zraika S, Beta-cell loss and beta-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Path. 2011. In press.
- Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, Pancreatic islet amyloidosis, beta-cell apoptosis, and alpha-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci U S A. 2009;106:13992–7.
- Maclean N, Ogilvie RF. Quantitative estimation of the pancreatic islet tissue in diabetic subjects. Diabetes. 1955;4:367–76.
- Gepts W. Die histopatohlogischen Veränderungen der Langerhansschen Inseln und ihre Bedeutung in der Frage der Pathogenese des menschlichen Diabetes. Endokrinologie. 1958;36:185–211.
- Klöppel G, Drenck CR. Immunzytochemische Morphometrie beim Typ-1- und Typ-2-Diabetes mellitus. Deutsch Med Wschr. 1983;108:188–9.
- Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978;15:417–21.
- Westermark P, Grimelius L. The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta Path Microbiol Scand A. 1973;81:291–300.
- Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diab Res. 1988;9:151–9.
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10 Suppl 4:32–42.
- Hoenig M. The cat as a model for human nutrition and disease. Curr Opin Clin Nutr Metab Care. 2006;9:584–8.
- Carlsson P-O. Influence of microenvironment on engraftment of transplanted β-cells. Ups J Med Sci. 2011;116:1–7.
- Andersson A, Borg H, Groth CG, Gunnarsson R, Hellerström C, Lundgren G, Survival of isolated human islets of Langerhans maintained in tissue culture. J Clin Invest. 1976;57:1295–301.
- Westermark GT, Westermark P, Nordin A, Törnelius E, Andersson A. Formation of amyloid in human pancreatic islets transplanted to the liver and spleen of nude mice. Ups J Med Sci. 2003;108:193–204.
- Westermark P, Andersson A, Westermark GT. Is aggregated IAPP a cause of beta-cell failure in transplanted human pancreatic islets? Curr Diab Rep. 2005;5:184–8.
- Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–9.
- Porte D Jr. Banting lecture 1990. β-cells in type II diabetes mellitus. Diabetes. 1991;40:166–80.