Abstract
Introduction. Previous studies have shown that a single sub-anesthetic dose of ketamine exerts fast-acting antidepressant effects in patients and in animal models of depression. However, the underlying mechanisms are not totally understood. This study aims to investigate the effects of acute administration of different doses of ketamine on the immobility time of rats in the forced swimming test (FST) and to determine levels of hippocampal brain-derived neurotrophic factor (BDNF) and mammalian target of rapamycin (mTOR).
Methods. Forty male Wistar rats weighing 180–220 g were randomly divided into four groups (n = 10 each): group saline and groups ketamine 5, 10, and 15 mg/kg. On the first day, all animals were forced to swim for 15 min. On the second day ketamine (5, 10, and 15 mg/kg, respectively) was given intraperitoneally, at 30 min before the second episode of the forced swimming test. Immobility times of the rats during the forced swimming test were recorded. The animals were then decapitated. The hippocampus was harvested for determination of BDNF and mTOR levels.
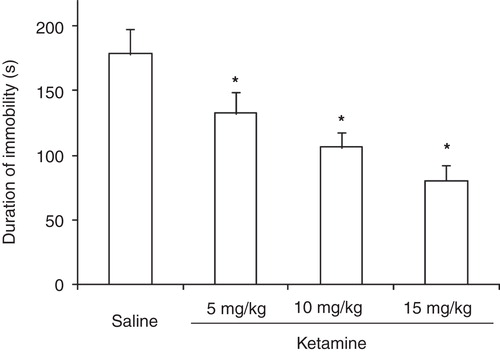
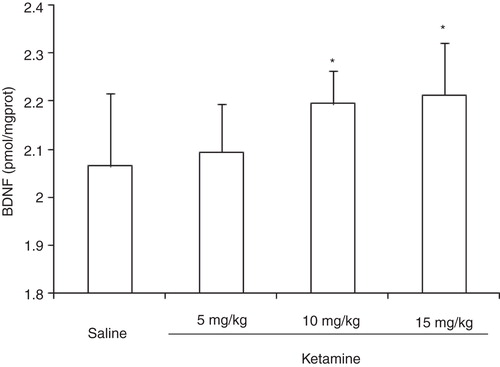
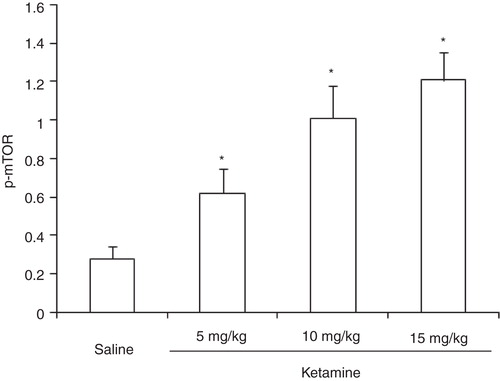
Results. Compared with group saline, administration of ketamine at a dose of 5, 10, and 15 mg/kg decreased the duration of immobility (P < 0.05 for all doses). Ketamine at doses of both 10 and 15 mg/kg showed a significant increase in the expression of hippocampal BDNF (P < 0.05 for both doses). Ketamine given at doses of 5, 10, and 15 mg/kg showed significant increases in relative levels of hippocampal p-mTOR (P < 0.05 for all doses)
Conclusion. The antidepressant effect of ketamine might be related to the increased expression of BDNF and mTOR in the hippocampus of rats.
Introduction
By now, the existing widely prescribed antidepressants are supposedly acting by an effect on monoamine systems. Monoamine-based antidepressants exert their therapeutic effects with a delayed onset of 2–4 weeks, and in some patients they do not have an effective improvement of mood even after a long time of treatment (Citation1). However, a large number of studies have shown that a single acute administration of a sub-anesthetic dose of ketamine, an ionotropic glutamatergic N-methyl-D-aspartate receptor (NMDAR) antagonist, produces a fast-acting and robust antidepressant effect both in patients suffering major depressive disorders (MDD) and in animal models of depression (Citation1,2). However, the underlying relevant mechanisms are not totally understood.
Levels of brain-derived neurotrophic factor (BDNF), an important neurotrophic factor, have been reported to be reduced in the central nervous system (CNS) and in peripheral blood in MDD (Citation3). It has also been shown that intra-cerebral infusion of BDNF results in an antidepressant effect in animal models of depression (Citation4). Moreover, exposure of rats to the forced swimming test (FST) reduces BDNF mRNA expression in some regions of the hippocampus, while antidepressant treatment increases the expression of BDNF mRNA in hippocampus (Citation5).
The mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase, which modulates cell growth, proliferation, motility, survival, and protein synthesis (Citation6). Li et al. (Citation7) have reported that an mTOR-induced rapid formation of synapses is an underlying mechanism for ketamine in exerting its rapid antidepressant effect. A recent case report has demonstrated that mTOR was constantly increased in peripheral blood of a depressed patient (Citation8). Moreover, in a post-mortem study it was reported that depressed patients are characterized by a deficit of mTOR in cerebral cortex (Citation9), which indicates that mTOR potentially might play an important role in the pathophysiology of depression.
Given the important roles of BDNF and mTOR in the treatment of MDD, the present study aims to investigate ketamine-induced antidepressant effects by analyzing the expression of hippocampal BDNF and mTOR after acute administration of different doses of ketamine in rats.
Methods
Animals
Forty male Wistar rats weighting 200–300 g were purchased from Shanghai Animal Center, Shanghai, China. The animals were housed five per cage with food and water available ad libitum, and were maintained on a 12 h light/dark cycle (lights on at 7.00 a.m.). Animals were involved in this experiment in accordance with the Guide for Care and Use of Laboratory Animals of National Institutes of Health.
Open-field test
An open-field test is usually performed to measure locomotor activity in rodents (Citation10). Briefly, the apparatus used in the present study consisted of a gray square 100 cm × 100 cm × 30 cm which was divided into 20 cm × 20 cm equal squares. Rats were placed in the center, the number of crossings (squares crossed with the four paws into one square) and rearings (posture sustained with hind-paws on the floor) were counted manually for 5 min. After each test the apparatus was cleaned.
Forced swimming test (FST)
This test included two separate exposures to a cylindrical tank with water in which rats cannot touch the bottom of the tank. The dimensions of the tank were 60 cm height and 30 cm diameter, and it was filled with water to a depth of 40 cm. The water in the tank was changed after the test of every rat. For the animal behavior observations, all procedures were conducted between 9.00 a.m. and 3.00 p.m. First, rats were placed in the water for 15 min without exposure to any drug. Twenty-three and a half hours later, rats were intraperitoneally injected with saline or different doses of ketamine. And 30 min later, rats were treated as the first episode for a 5 min session, and the immobility time was recorded. The definition of immobility in the FST is that the rat remained floating in the water without struggling and made only movements necessary to keep its head above the water (Citation11). Rats were then intraperitoneally injected with saline or different doses of ketamine 30 min later, and a second episode of FST was then carried out. Counting immobility time was conducted by the same trained observer.
Test of BDNF
After the FST, animals were killed immediately, and their hippocampi dissected. BDNF levels in the hippocampi were measured by anti-BDNF sandwich-ELISA, according to the manufacturer's instructions (Chemicon, Billerica, MA, USA). Briefly, rat hippocampus was homogenized in phosphate buffer solution with 1 mM phenylmethylsulfonyl fluoride and 1 mM EGTA. Microtiter plates (96-well flat-bottom) were coated for 24 h with the samples diluted 1:2 in sample diluent. The standard curve ranged from 7.8 to 500 pg/mL of BDNF. The plates were then washed four times with sample diluent, and a monoclonal anti-BDNF rabbit antibody diluted 1:1000 in sample diluent was added to each well and incubated for 3 h at room temperature. After washing, a peroxidase-conjugated anti-rabbit antibody (diluted 1:1000) was added to each well and incubated at room temperature for 1 h. After addition of streptavidin enzyme, substrate and stop solution, levels of BDNF were determined by absorbance at 450 nm. The standard curve demonstrated a direct relationship between optical density and BDNF concentration. Total protein was measured by Lowry's method using bovine serum albumin as a standard.
Test of mTOR
Rat hippocampus was directly homogenized in ice-chilled buffer. Samples of homogenates (15 μg of protein) were subjected to SDS-PAGE under reducing conditions. Proteins were transferred onto PDVF membranes, which were incubated with anti-phospho mTOR Ser 2448 (1:2000, Cell Signaling Company, Beverly, MA, USA).
Statistical analysis
Data are expressed as mean ± standard deviation (SD). Statistical analyses were made by one-way analysis of variance, and post-hoc analyses were performed by least significant difference tests. These statistical analyses were conducted by Statistical Product for Social Sciences (SPSS version 17.0). Differences were considered to be significant at P < 0.05.
Results
Locomotor activity
Number of crossings showed no statistically significant differences between groups, and neither did number of rearings show any differences in the open-field test (P > 0.05 for all doses) (detailed data not shown).
Duration of immobility
There were statistically significant differences between the different test groups with regard to duration of immobility (F (3,36) = 77.64, P < 0.01). Thus, administration of ketamine at doses of 5, 10, and 15 mg/kg decreased the duration of immobility significantly (P < 0.05 for all doses) ().
Expression of BDNF
The expression of hippocampal BDNF showed significant differences among groups (F (3,36) = 4.28, P < 0.05). While administration of ketamine at doses of 10 and 15 mg/kg showed a significant increase in the expression of hippocampal BDNF (P < 0.05), ketamine at a dose of 5 mg/kg presented no significant effect on the expression of hippocampal BDNF (P > 0.05) ().
Discussion
Previous studies usually put forward the opinion that administration of ketamine has inherent side effects, such as hallucinations and schizophrenia-like symptoms, especially at large doses (Citation12-15). Therefore, we chose acute administration of sub-anesthetic doses of ketamine to investigate its antidepressant effect. In this study, we tested the locomotor activity by the open-field test, and the results showed that ketamine did not induce significant changes with regard to horizontal and vertical movements, suggesting that ketamine has no influential effects on the duration of immobility in rats.
Depression is characterized as an increased immobility time in rats subject for FST as observed in the second episode of the two tests separated by 24 h (Citation16,17). In the present study, acute administration of ketamine to rats decreased the immobility time and increased the expression of BDNF and mTOR in their hippocampi. Compared with conventional antidepressants, ketamine exerts a rapid-acting antidepressant effect within 0.5–2 h after administration in animal models (Citation18). However, Popik et al. (Citation19) have, shown that ketamine does not exert a persistent antidepressant effect in rodent models. Most of existing clinical studies support the opinion that ketamine has an ability to ameliorate the symptoms of depressed patients, especially in patients with treatment-resistant depression (Citation20,21). Our results demonstrate that ketamine decreases the duration of immobility as measured in the rodent FST, supporting the idea that ketamine indeed has got an antidepressant effect.
The role of BDNF in the CNS has been implicated in the etiology of depression and in the action of antidepressant agents (Citation22). In support of this, we observed increased levels of BDNF in the rat hippocampus after ketamine treatment. Interestingly, we observed that acute administration of ketamine at a dose of 10 mg/kg increased the expression of BDNF, whereas 5 mg/kg did not. Our results are in line with those of Garcia et al. (Citation23) who have shown that acute administration of ketamine at a dose of 15 mg/kg increased the hippocampus BDNF levels. However, in another study of Garcia et al. (Citation25) it was shown that chronic administration of ketamine elicited an antidepressant effect in rodents without affecting the BDNF levels in hippocampus. The different expressions of the BDNF between the acute and chronic administration suggested that some other signaling pathways also mediate ketamine-induced antidepressant effect.
Since Li et al. (Citation7) found that mTOR plays an important role in the mechanisms of ketamine exerting its fast-acting antidepressant effect, several subsequent studies have focused on the role of this enzyme. Clinical research has shown that mTOR is likely to be implicated in the etiology of depression and in the antidepressant mechanism of ketamine (Citation8,9). Our results indicate that acute administration of increasing doses of ketamine elicited a gradually enhanced antidepressant effect associated with an increased mTOR expression in rat hippocampus.
The coincidence of the decrease of immobility time and the increase of BDNF and mTOR after ketamine treatment suggests that there is an underlying link between antidepressant effects and the increase of BDNF and mTOR. Recently, Li et al. (Citation26) have shown that ketamine, in a rat model, possibly exerts its antidepressant effects via rapid neurogenesis. Moreover, BDNF and mTOR are both important promoters for neurogenesis (Citation27,28). In the present study, the levels of hippocampal BDNF and mTOR increased 30 min after administration of ketamine. Therefore, we speculate that the antidepressant effect of ketamine may be attributed to a rapid neurogenesis induced by an increase of both BDNF and mTOR.
Acknowledgements
This study was supported by a grant from the National Natural Science Foundation of China (Grant No. 30872424).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Machado-Vieira R, Salvadore G, Diazgranados N, Zarate CA Jr. Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacol Ther. 2009;123:143–50.
- Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64.
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–57.
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol Biochem Behav. 1997;56:131–7.
- Russo-Neustadt A, Ha T, Ramirez R, Kesslak JP. Physical activity-antidepressant treatment combinations: impact on brain-derived neurotrophic factor and behavior in an animal model. Behav Brain Res. 2001;120:87–95.
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2006;18:1926–45.
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;29:959–64.
- Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck C. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiatry. 2011;168:751–2.
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–9.
- Sherif F, Oreland L. Effect of the GABA-transaminase inhibitor vigabatrin on exploratory behaviour in socially isolated rats. Behav Brain Res. 1995;72:135–40.
- Liu L, Li Q, Sapolsky R, Liao M, Mehta K, Bhargava A, Transient gastric irritation in the neonatal rats leads to changes in hypothalamic CRF expression, depression- and anxiety-like behavior as adults. PLoS One. 2011;6:e19498.
- Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H. Glutamatergic (N-methyl-D-aspartate receptor) hypofrontality in schizophrenia: too little juice or a miswired brain? Mol Pharmacol. 2010;77:317–26.
- Pomarol-Clotet E, Honey GD, Murray GK, Corlett PR, Absalom AR, Lee M, Psychological effects of ketamine in healthy volunteers. Phenomenological study. Br J Psychiatry. 2006;189:173–9.
- Roberts BM, Shaffer CL, Seymour PA, Schmidt CJ, Williams GV, Castner SA. Glycine transporter inhibition reverses ketamine-induced working memory deficits. Neuroreport. 2010;21:390–4.
- Hunt MJ, Raynaud B, Garcia R. Ketamine dose-dependently induces high-frequency oscillations in the nucleus accumbens in freely moving rats. Biol Psychiatry. 2006;60:1206–14.
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2.
- Yilmaz A, Schulz D, Aksoy A, Canbeyli R. Prolonged effect of an anesthetic dose of ketamine on behavioral despair. Pharmacol Biochem Behav. 2002;71:341–4.
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–11.
- Popik P, Kos T, Sowa-Kućma M, Nowak G. Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology (Berl). 2008;198:421–30.
- DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–11.
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–6.
- Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–97.
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–4.
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–6.
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol. 2008;103:502–6.
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61.
- Furukawa-Hibi Y, Nitta A, Ikeda T, Morishita K, Liu W, Ibi D, The hydrophobic dipeptide Leu-Ile inhibits immobility induced by repeated forced swimming via the induction of BDNF. Behav Brain Res. 2011;220:271–80.
- Han J, Wang B, Xiao Z, Gao Y, Zhao Y, Zhang J, Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–24.