Systemic sclerosis (SSc) can be a devastating disease with lethal complications such as interstitial lung disease (ILD). Therapeutic strategies include cyclophosphamide but patients are frequently refractory even to this aggressive immunosuppressant. Lafyatis et al published a 6-month open observational study of 15 patients with diffuse scleroderma receiving one course of rituximab (2 × 1 g within 2 weeks) (1, 2). They found no significant clinical improvement in the extent of thickened skin and no significant benefit in pulmonary functional tests. Daoussis et al published a 1-year randomized controlled trial, in which eight scleroderma patients improved on skin fibrosis and pulmonary function (2, 3). This success was prolonged in an open 2-year observation of patients receiving four weekly pulses of 375 mg/m2 rituximab after 6, 12, and 18 months (2, 4).
We report here a significant benefit with a different dosing regimen in a small open series of scleroderma patients with ILD.
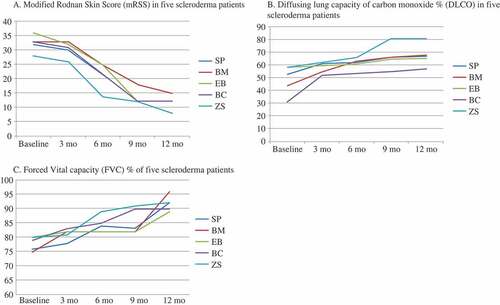
Five consecutive patients with anti-topoisomerase I (Scl-70)-positive diffuse scleroderma and ILD failed to respond to conventional therapy with intravenous cyclophosphamide. Demographic data and patients’ characteristics are shown in a Table (attached as supplementary material). All five patients received 500 mg rituximab on day 0 and day 14 every 3 months for a period of 1 year. Changes in the modified Rodnan skin score (mRSS) and diffusion lung capacity of carbon monoxide (DLCO) are shown in .
Figure 1. Changes in (A) the modified Rodnan skin score (mRSS), (B) the diffusing capacity of the lung for carbon monoxide (DLCO), and (C) forced vital capacity (FVC) measurements in all five scleroderma patients (SP, BM, EB, BC, and ZS) treated with rituximab at 0, 3, 6, and 12 months. Changes in mRSS and DLCO are shown as percentages for up to 12 months of treatment with rituximab. There was a significant decrease (mean ± SD) in mRSS in all five patients (26.5 ± 8.3% vs. 11.8 ± 2.9%, p < 0.001). DLCO improved after the therapy with rituximab in all patients (48.5 ± 6.7% vs. 66.0 ± 4.0%, p < 0.001). FVC improved significantly after 6 months (72 ± 5.2% vs. 82 ± 5.9%, p < 0.008) and 12 months (72 ± 5.2% vs. 89 ± 3.2%, p < 0.004).
In three patients descriptive quantitation of the extent of lung fibrosis by our radiologist (AL) was available. This decreased substantially as shown in Figure 2 (attached as supplementary material). Moreover, in all five patients digital ulcerations healed and the severity of Raynaud’s phenomenon and vascular pain decreased. The number of capillary bleeds and megacapillaries decreased as shown by capillary microscopy. The B-lymphocyte count decreased, as expected, in all patients after rituximab treatment, while there was no change in the concentration of serum immunoglobulins, autoantibody titres, or C-reactive protein (CRP) levels (5).
Our clinical experience matches the observations that rituximab may be a promising drug to control the progression of lung involvement in SSc (1–7).
The issue of dosing of rituximab in scleroderma needs to be examined in longitudinal and controlled studies. In rheumatoid arthritis, a meta-analysis showed no significant difference in outcome regarding the used dosage of rituximab (2). The common single application of glucocorticoids prior to rituximab infusion does not seem to be of relevance for our patient outcomes because similar amounts of glucocorticoids had also been administered during previous cyclophosphamide treatment in all five cases.
In summary, we demonstrate in an uncontrolled series of five patients that rituximab was able to improve skin thickness and lung function in severe progressive SSc refractory to immunosuppressive therapy with cyclophosphamide. It seems appropriate to test the effect of dosage, co-medication, and the time course of rituximab application in controlled and longitudinal studies.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Table 1: Demographic data of the five diffuse scleroderma patients treated with rituximab
Figure 2: High resolution computed tomography (HRCT) of the lung in patients with scleroderma
Please note: The editors are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries should be directed to the corresponding author for the article.
Supplementary Material
Download PDF (168.6 KB)Acknowledgements
We thank E. Lamont for language editing.
References
- Lafyatis R, Kissin E, York M, Farina G, Viger K, Fritzler MJ B cell depletion with rituximab in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum 2009;60:578–83.
- Bredemeier M, de Oliveira FK, Rocha CM. Low- versus high-dose rituximab for rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). Published online: 27 August 2013. doi: 10.1002/acr.22116.
- Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rheumatology (Oxford) 2010;49:271–80.
- Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Paliogianni F, Sirinian C Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol 2012;30:S17–22.
- Diot E, Boissinot E, Asquier E, Guilmot JL, Lemarie E, Valat C Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest 1998;114:1623–9.
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004;350:2572–81.
- Daoussis D, Liossis SN, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Korfiatis P Is there a role for B-cell depletion as therapy for scleroderma? A case report and review of the literature. Semin Arthritis Rheum 2010;40:127–36.
