Abstract
For more than 3500 years, urinary catheters have been used to drain the bladder when it fails to empty. For people with impaired bladder function and for whom the method is feasible, clean intermittent self-catheterization is the optimal procedure. For those who require an indwelling catheter, whether short- or long-term, the self-retaining Foley catheter is invariably used, as it has been since its introduction nearly 80 years ago, despite the fact that this catheter can cause bacterial colonization, recurrent and chronic infections, bladder stones and septicaemia, damage to the kidneys, the bladder and the urethra, and contribute to the development of antibiotic resistance. In terms of medical, social and economic resources, the burden of urinary retention and incontinence, aggravated by the use of the Foley catheter, is huge. In the UK, the harm resulting from the use of the Foley catheter costs the National Health Service between £1.0–2.5 billion and accounts for ∼2100 deaths per year. Therefore, there is an urgent need for the development of an alternative indwelling catheter system. The research agenda is for the new catheter to be easy and safe to insert, either urethrally or suprapubically, to be retained reliably in the bladder and to be withdrawn easily and safely when necessary, to mimic natural physiology by filling at low pressure and emptying completely without damage to the bladder, and to have control mechanisms appropriate for all users.
1. A brief history of the development of the urinary catheter
The word catheter is derived from the ancient Greek kathiénai, which literally means “to thrust into” or “to send down”.
The main events in the chronology of the recorded development of the urinary catheter are identified in . Before the widespread introduction of the Foley catheter [Citation14] in the 1930s, catheterization was almost exclusively for the treatment of urinary retention in the male. The early catheters (some examples are shown in ) were usually rigid and they were designed—to the extent that they were designed at all—for intermittent catheterization. shows what was involved, although most sufferers presumably would have preferred a greater degree of privacy. Urinary retention was—and is—rare in women. Urinary incontinence was not a medical emergency: it was left as a personal embarrassment for men and women alike, who generally adopted their own idiosyncratic methods of coping with the disability. The indwelling Foley catheter, however, made both short- and long-term catheterization feasible for both males and females and this opened up a new era in the management of urinary retention and incontinence. It also opened up a Pandora’s box of medical conditions and adverse events.
Figure 1. Tubular silver catheters devised by Ambroise Paré (1510–1590), with long gentle curves (they are known as coudé catheters) to permit easier insertion [Citation8].
![Figure 1. Tubular silver catheters devised by Ambroise Paré (1510–1590), with long gentle curves (they are known as coudé catheters) to permit easier insertion [Citation8].](/cms/asset/cb80016f-8a8a-4666-a3ad-29c960496f29/ijmt_a_1085600_f0001_b.jpg)
Figure 2. Urinary catheterization in the middle ages [Citation17].
![Figure 2. Urinary catheterization in the middle ages [Citation17].](/cms/asset/fab0c956-15cf-4d69-a821-fe955089d3b1/ijmt_a_1085600_f0002_b.jpg)
Table 1. Some important events in the history of the development of the urinary catheter.
2. Bladder function and catheterization
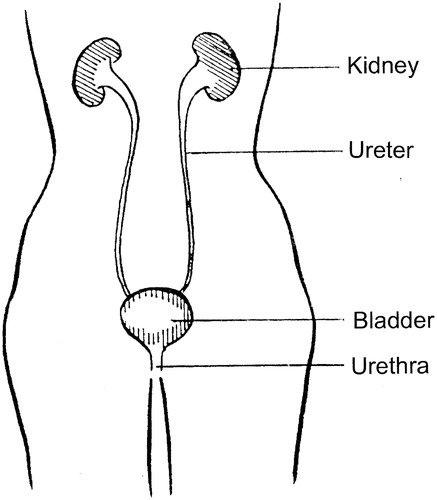
The urinary tract system () produces, stores and excretes urine from the body. In the adult, under normal conditions of hydration and temperature, the kidneys continuously filter the blood to produce ∼1 ml of urine per minute, equivalent to ∼1500 ml per day. Urine from the kidneys is transported via the ureters to the bladder. The capacity of the bladder is variable: a healthy bladder can normally hold 350–500 ml. Three sets of muscles control the flow of urine from the bladder via the urethra. The internal sphincter is formed by the involuntary smooth muscle of the bladder wall, located at the base of the bladder where it joins the urethra. The external sphincter which surrounds the proximal part of the urethra is formed by striated muscle and is, thus, under voluntary control. Third, the pelvic floor muscles act as a sling to support the bladder and urethra and provide additional control. Urine flow is initiated by the voluntary relaxation of the external sphincter muscle which, by reflex action, triggers contraction of the bladder muscle and opening of the internal sphincter.
In the normal urinary tract, the regular flushing of the urethra as the bladder empties helps to impede the ascending infection of the tract by the bacteria that normally colonize the periurethral skin. Any bacteria that manage to migrate into the bladder are also washed out during micturition. In addition, the bladder is lined by urothelial cells coated with a glycosaminoglycan mucin, which provides a surface resistant to the adherence of bacteria. Bacterial adherence, when it does occur, initiates invasion of the urothelium. This activates microbial-sensing proteins in the superficial umbrella cells, triggering the host defences with a cascade of cellular and molecular effectors to eliminate the bacteria [Citation18].
In people with impaired bladder function, whether retention or incontinence, a safe and reliable system is required to collect and contain the urine, whether for short- or long-term use.
In those males and females for whom the method is feasible, clean intermittent self-catheterization is the optimal procedure to manage urinary retention [Citation19]. This mimics normal bladder function, allowing the bladder to fill and periodically to empty completely, thus minimizing the risk of infection. Although some find the procedure uncomfortable and distasteful, with practice clean intermittent self-catheterization is usually quite easy to perform. Whilst observing a high standard of cleanliness, the patient inserts a flexible catheter (typically a plastic tube, with drainage eyes adjacent to its rounded closed tip) into the urethra until urine starts to flow, drains the urine directly into a toilet bowl or into a suitable container for later disposal and withdraws the catheter when the flow ceases. The procedure needs to be repeated six or seven times a day, depending on the volume of residual urine. In the UK, catheters tend to be used only once, but randomized controlled trials are being undertaken to assess whether multiple uses of the same catheter might be acceptable. In a study of 172 adults (68 male, 104 female), seven were unable or unwilling to adopt the technique, but, for the remainder, the mean infection rate was only one per 14 patient-months [Citation20].
In patients for whom clean intermittent self-catheterization is not possible, an indwelling catheter has to be used. Depending on the clinical indication, the duration of catheterization may be short- or long-term. A long-term urinary catheter is defined as one that is in place for more than 30 days.
For male patients with urinary incontinence, one possibility for short- or long-term use is the external or condom catheter. This consists of a sheath that fits snuggly over the penis and which has a tube at its tip to transport urine to a collection bag, which may be strapped to the leg and emptied periodically. Although this is superficially an attractive approach, it does have several significant disadvantages. Up to 40% of condom catheter users develop urinary tract infections [Citation21], 15% suffer from inflammation, ulceration, necrosis, gangrene or constriction of the skin of the penis [Citation22] and there is the ever-present risk of detachment of the condom and urine leakage. Moreover, the nursing time required for condom catheter care is considerable [Citation23]. In summary, the condom catheter is far from satisfactory in the management of male urinary incontinence; it does, however, have a useful application in the non-invasive measurement of bladder pressure [Citation24].
The eponymous indwelling balloon-retained catheter () now in worldwide use was conceived by the American urologist Frederic Foley nearly 80 years ago to provide continuous urinary drainage and to control bleeding while haemostasis occurs, following transurethral prostatectomy [Citation14]. It soon became apparent, however, that the use of the Foley catheter was a solution to the general problems of urinary retention and incontinence. From the outset, buckets or open flasks were used for urine collection: it was not until the 1960s that a bag that could be strapped to the patient’s leg was introduced as an hygienic and more aesthetically acceptable alternative.
Figure 4. A typical Foley catheter. This catheter is size 16 Fr. Its overall length is ∼400 mm and the volume of the fully-inflated balloon is ∼10 ml. The catheter has two channels. When the catheter has been inserted, the retaining balloon is inflated with sterile water from a syringe via the inflation connector and one of the channels. The inflation connector incorporates a valve to prevent the sterile water from escaping when the syringe is detached. The other channel allows the free flow of urine from the drainage eye to the drainage funnel. To remove the catheter, the retaining balloon is first deflated by withdrawing the water from it with a syringe, which opens the valve in the inflation connector when it is attached.
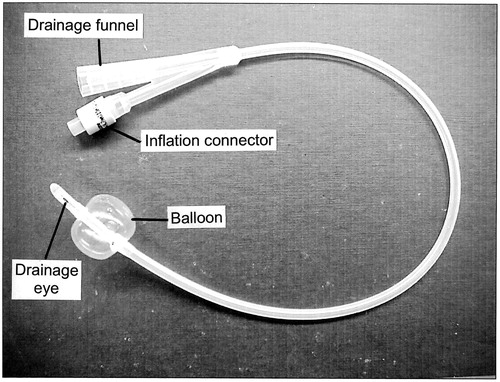
The design of the Foley catheter is simple. As shown in , the catheter typically has two channels, the drainage channel for the passage of urine and the inflation channel, to allow the balloon at the end of the catheter to be inflated with sterile water from a syringe, to retain the catheter within the bladder. The smooth rounded tip of the catheter extends beyond the balloon and one or more eye-holes are cut in the tube adjacent to the tip to allow urine to drain. Were it not for its complications (discussed in Section 4), it would often be desirable for a Foley catheter to be able to be in place for up to ∼12 weeks before the possibility of mechanical failure of the balloon would dictate its replacement.
The principal reasons for indwelling catheterization are as follows:
to permit urinary drainage in patients with neurological conditions which cause bladder dysfunction;
to manage urinary incontinence in patients lacking cognitive function;
to minimize skin breakdown and pressure ulcers in paralysed, comatose or terminally ill patients;
to irrigate the bladder;
to administer chemotherapy;
to aid in urological surgery or other surgery on contiguous structures;
to obtain accurate measurements of urinary output in critically ill or post-operative patients;
to empty the bladder during childbirth; and
to undertake urodynamic studies (such as pressure measurements).
Of these, (a), (b) and (c) are likely to be for long-term catheterization.
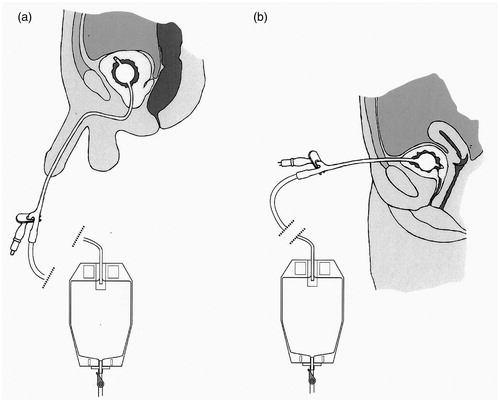
Bladder drainage may be performed by passing a Foley catheter through the natural urethral passage (termed transurethral catheterization) or by creating an artificial track between the lower abdominal wall and the bladder (suprapubic catheterization), as shown in . Transurethral catheterization is the simpler and safer approach. The female urethra is short, being ∼40 mm in length, muscular and straight. The male urethra is ∼160 mm long, more sensitive and curved, which can give rise to complications. Passage of a catheter can be painful and, in the male, the curvature of the urethra introduces a risk that its tip may cause damage [Citation25]. Some designs of catheters are curved (coudé and bicoudé) to minimize this risk. The main problem with suprapubic catheterization is the risk of perforating the bowel on insertion of the guidance cannula [Citation26]. Guidelines on minimizing morbidity associated with suprapubic catheter usage have been published by the British Association of Urological Surgeons [Citation27].
Figure 5. The Foley catheter, introduced (a) Urethrally and (b) Suprapubically. In both cases, the bladder is shown to be draining continuously into a urine collection bag attached to the leg: this bag can be emptied when necessary by opening a valve. Alternatively, the bladder can be drained intermittently if a catheter valve is inserted into the drainage funnel of the catheter.
Catheter size is usually expressed in French gauge (Fr or FG = circumference in mm). The normal practice is to use the smallest catheter compatible with good drainage [Citation28]: 12–16 Fr is usually adequate and only rarely is a catheter larger than 18 Fr necessary.
3. The modern Foley catheter
Foley’s original catheter was made of latex, the mechanical properties of which are ideal for this purpose: it has a high stretch ratio, a high level of resilience and it is extremely waterproof. The main problem with latex is its cytotoxicity: for instance, in the 1980s, an epidemic of severe urethral strictures was recorded in patients as the result of using latex catheters. The cause was traced to cellular toxicity due to eluates from rubber [Citation29]. Latex catheters are now usually coated with silicone elastomer to reduce this risk [Citation30]. Many modern catheters are made entirely of silicone elastomer and hydrophilic coatings are used to provide a slippery surface to reduce friction [Citation31]. Silicone catheters are not only non-allergenic, but they also have superior resistance to kinking and better flow properties in comparison with latex catheters [Citation32].
Emphasis has also been placed on the need for a smooth surface to the catheter and the drainage eyes [Citation33]. Rough surfaces encourage the deposition of bacterial biofilm and sharp edges to the drainage eyes can cause bleeding from the urethral lining when the catheter is introduced or withdrawn.
Some catheter research over the last few years has focused on the development of antiseptic and antimicrobial coatings, with the aim of reducing the incidence of catheter-associated urinary tract infections [Citation34], so far with negligible success [Citation35] (see also Section 6d). Thus, a randomized controlled trial performed to compare the ability and cost-effectiveness of an antiseptic- and antimicrobial-impregnated catheter vs a standard coated catheter to minimize the risk of catheter-associated urinary tract infection revealed no evidence of benefit [Citation36]. Indeed, in an earlier randomized clinical trial, infection actually increased with a silver-impregnated catheter [Citation37].
Some Foley catheters have a third channel, which can be used to infuse saline or other irrigating fluid into the bladder: this may be useful when there is a likelihood that blood clots may form in the bladder, perhaps as the result of post-operative bleeding. There is also a commercially-available catheter that has two balloons at the end of the catheter. The balloon at the tip is intended to reduce the risk of trauma to the urothelium; the drainage eyes perforate a short section of catheter between the two balloons, the proximal of which serves as the retention device. A possible disadvantage of the dual-balloon catheter is that it may trap more urine in the bladder at the end of drainage, thus increasing the risk of bladder infection.
4. Adverse events caused by the Foley catheter
In an editorial in the New England Journal of Medicine in 1988, the American physician, Calvin M. Kunin, published a comprehensive indictment of the Foley catheter, 51 years after its introduction into clinical practice [Citation38]. Having acknowledged that the Foley catheter is indispensable in modern clinical practice to provide temporary relief of urinary retention, a dry environment for incontinent or comatose patients and an accurate measurement of urinary output in those who are seriously ill, his intervention added momentum to the publication of major contributions covering virtually every aspect of the subject.
An adverse event is defined as “any untoward medical occurrence, unintended disease or injury or any untoward clinical signs in subjects, users or other persons”[Citation39]. For an adverse event to be considered to be serious, it should either: have led to a death; or have led to a serious deterioration in health that either resulted in life-threatening illness or injury or permanent impairment of a body structure or body function, or required in-patient hospitalization or prolongation of existing hospitalization or resulted in medical or surgical intervention to prevent life-threatening illness or injury or permanent impairment of a body structure or a body function; or led to foetal distress, foetal death or a congenital abnormality or birth defect [Citation39]. The principal adverse events—some of which are serious—for which the Foley catheter is responsible are as follows:
4.1. Bacterial colonization
The flow of urine through an indwelling catheter may be continuous or intermittent. The introduction of a Foley catheter without a valve results in continuous drainage and, thereby, suppresses the normal process by which the build-up of bacteria is inhibited by periodic flushing. Periodic flushing is usually facilitated by a manually-operated pinch or rotary valve. It can also be provided by “tidal drainage”, which allows the bladder to fill and empty automatically [Citation40]. By raising the height of the drainage tubing leading from the catheter to a few centimetres above the level of the bladder, the bladder fills to the corresponding hydrostatic pressure before a syphon is formed that empties the bladder, after which the cycle is repeated. In a series of 33 patients with neurologically-damaged bladders following spinal cord injuries, tidal drainage reduced the rate of infection from 73% to 15% [Citation41]. Although this is a marked improvement and urethral damage can be avoided by suprapubic catheterization [Citation42], tidal drainage is seldom used nowadays, possibly because of the level of nursing care required.
Bacteria can invade the bladder by migrating along the inside and the outside of the catheter. With short-term catheterization, the daily infection rate is ∼5% [Citation16], so that ∼95% of catheterized patients suffer bacterial invasion after 1 month. Urinary tract infection necessitates the use of antibiotics, which are all too frequently untested against the specific bacteria and consequently often prove to be ineffective until the right one is found by a process of trial and error. This adds to the cost of clinical management, as well as being a burden for patients and carers.
4.2. Antibiotic resistance
The use of antibiotics to control catheter-induced infections contributes significantly to the development of resistant strains, about which the World Health Organization (WHO) has expressed grave concern [Citation43]. The WHO referred in particular to seven bacteria: the first of these, Escherichia coli, is strongly associated with urinary tract infections. In five out of the six WHO regions, it was found that the antimicrobial drugs that were used failed in 50% or more of the cases investigated. The second bacterium in the list, Klebsiella pneumonia, which is also found in urinary tract infections, was similarly resistant. In view of the increasingly serious threat to global public health identified by the WHO, the present pervasive lack of interest in research aimed at finding a better alternative to the Foley catheter is both disturbing and inexcusable.
4.3. Chronic infection
The balloon of the Foley catheter occupies the base of the bladder, obstructing the internal urethral orifice, with the result that 10–100 ml of urine remains in the bladder when its flow has ceased [Citation44]. This sump of residual urine is likely to be infected, so that uninfected urine descending from the kidneys will also rapidly become infected, resulting in chronic infection of the bladder.
4.4. Kidney and bladder damage
Invasion of the bladder by urease-producing bacteria, particularly Proteus mirabilis, results in the conversion of urea in the urine into ammonia [Citation35]. The consequential increase in the alkalinity of the urine causes phosphates to nucleate out of solution, forming crystals of struvite (magnesium ammonium phosphate) and hydroxyapatite (an hydroxylated form of calcium phosphate in which some of the phosphate groups are replaced by carbonate). Increasing fluid intake with citrate-containing drinks increases the pH at which crystals form in the urine [Citation45] and there is evidence [Citation46,Citation47] that this could be used to control the rate at which catheter encrustation occurs.
The nucleation of struvite and hydroxyapatite crystals on the biofilm on the catheter resulting from the activity of the bacterial urease causes encrustation to form around and within the catheter, blocking the drainage eyes and the lumen and preventing the flow of urine (). This is a medical emergency that not only can be excruciatingly painful for the patient, but that also and more importantly requires a rapid response (usually the replacement of the blocked catheter) if permanent damage to the bladder and the kidneys (due to ureteric reflux) caused by the high bladder pressure is to be avoided. These problems are exacerbated if associated with bladder spasm [Citation48]. Moreover, it is apparent that some patients are more likely to block their catheters than others [Citation49] (blockers tend to have urine that is more alkaline, which is consistent with other observations).
Figure 6. A section through a Foley catheter that has become blocked during use by the formation of struvite. The smaller patent lumen is the channel for the inflation and deflation of the retaining balloon. The length of the scale bar is 1 mm.

It has been reported that, in cases of Proteus mirabilis infection, the necessity for antibiotics might be avoided by adding the biocide triclosan to the sterile water used to inflate the balloon of the Foley catheter. This appears to prevent the rise in urinary pH that drives biofilm formation and catheter blockage [Citation50], presumably by leaching into the urine. It is disappointing, however, that exposure to triclosan has also been shown to encourage the development of resistant strains of Proteus mirabilis, so this does not hold out much promise as a long-term solution [Citation51].
4.5. Bladder stones
The crystals of struvite resulting from Proteus mirabilis infection act as nuclei for stone formation within the bladder [Citation52]. Bladder stones entrap Proteus mirabilis bacteria and, thus, maintain the infection. Recurrent blockage of a catheter raises a high suspicion that bladder stones may be present. Endoscopic transurethral techniques are used to remove bladder stones: fragmentation by crushing (litholapaxy), shock-wave ultrasound or laser probes may be required to break them into particles small enough to be washed out of the bladder through the urethra.
4.6. Pseudopolyps
Insertion into the urethra of a hard unyielding catheter, with its balloon and its protruding tip perforated by drainage eyes, transforms the natural process of intermittent drainage. When the drainage valve at the distal end of the catheter is opened, the low viscous drag of the catheter allows the urine to flow rapidly, driven by both the collapsing bladder and the negative pressure of the hydrostatic column to the open end of the catheter. Towards the end of the drainage process, when the bladder wall comes into what is frequently traumatic contact with the tip of the catheter, the mucosal lining can be sucked into the drainage eye [Citation53]. Patients may experience a sharp pain at this stage, sometimes accompanied by “stuttering” when the urine flow momentarily ceases as the result of the bladder wall being sucked into the drainage eye and then released. This suction can result in the formation of haemorrhagic pseudopolyps, with cumulative damage [Citation54].
4.7. Septicaemia
The physical trauma caused by the catheter tip and the suction at the drainage eyes can damage the normally impermeable bacterial barrier provided by the urothelial lining of the bladder (see Section 4.6). This provides direct access for bacteria into the bladder wall and the bloodstream (bacteraemia), with a high risk of septicaemia. Moreover, reflux of infected urine via the ureters can lead to renal infection (pyelonephritis) and septicaemia. If inadequately treated, septicaemia can prove to be fatal [Citation55].
4.8. Urethral trauma
The process of inserting the catheter requires skill and practice, if urethral trauma is to be avoided [Citation56]. One of the problems with indwelling catheters with silicone balloons is that, when the water is removed from the balloon with a syringe prior to catheter withdrawal, a phenomenon known as creep may cause the balloon to fail to collapse completely. This may result in a small rim that can make it difficult or impossible to to remove the catheter [Citation57]. (This presents a particular problem in the case of suprapubic catheters because these pass through a rigid fibrous track into the bladder, rather than through the urethra with its more elastic muscular walls.) Even more serious damage can occur if the catheter is deliberately pulled out when the balloon is still inflated, as can be done by disorientated or demented patients. In women with neurological conditions such as multiple sclerosis, the catheter can be expelled spontaneously by a sudden inappropriate contraction of the bladder. Under these circumstances, the urethra is dilated by the balloon and, if frequently repeated, the sphincter mechanism may become incompetent.
4.9. Balloon fragments
There is the risk that the catheter balloon may burst, either during insertion or withdrawal (particularly by a demented or disorientated patient) or when it is indwelling [Citation58]. If this should happen, the fragments must be removed, usually with the aid of a cystoscope, as otherwise they can lead to stone formation or catheter blockage.
4.10. Commentary
Several of these adverse events are demonstrated in a video presentation that can be accessed via the internet [Citation59].
It is axiomatic that the incidence of catheter-associated urinary tract infections can be reduced by reducing unnecessary catheter use. There have been numerous studies aimed at achieving this worthy objective. In reviews of these studies [Citation60,Citation61], two effective strategies have been identified. First, unnecessary placement of indwelling catheters should be avoided by imposing protocols including, for instance, a requirement to confirm by ultrasonic scanning that urine is being retained in the bladder [Citation62]. There are semi-automated scanners designed for this purpose, but they are not without their shortcomings [Citation63]. Second, there should be a system to remind the medical and nursing staff to be aware of the catheter’s existence, perhaps by means of a daily checklist, to prompt its removal when no longer necessary. This simple expedient can have a significant impact.
The achievable goal of the implementation of these two strategies is typically to reduce the rates of catheter-associated infections by 25% [Citation60]. These strategies are already being implemented in many hospitals but, even if they were universally followed, the Foley catheter would still have to remain in widespread use.
5. The scale of the burden of urinary incontinence
5.1. Overview
Data from 2006–2007 reveal that ∼1.3 million people in England sought help for incontinence problems. The number had risen to 2.3 million in 2010–2011. Urinary incontinence increases with age from 14% in individuals aged 65–69 years to 45% in those aged 85 years or over [Citation64]. The care of older and disabled people in an ageing population presents a major challenge: the management of bladder (and bowel) function is fundamental to the standard of care that they receive. It is difficult or impossible for those affected to maintain a reasonable quality-of-life and urinary incontinence is a major reason for sufferers to seek residential care [Citation65].
In a study of 430 new admissions to nursing homes in the US, 39% of patients aged 65 years or over suffered from daytime urinary incontinence [Citation66]. In this setting, catheterization is only recommended as a last resort, because of its high incidence of urinary tract infections. Elderly patients are managed by using incontinence pads, but immobile patients lying on wet pads develop pressure sores. Choosing the lesser of two evils, the development of a pressure sore is accepted as an indication for catheterization.
In England, Wales and Northern Ireland (and there is no reason to suppose that the situation is significantly different in Scotland), indwelling Foley catheters are used by 3% of people living in the community and 13% of care home residents [Citation67].
5.2. Incidence of adverse events
It is a profoundly disturbing statistic that healthcare-associated urinary tract infections are estimated to have caused 13 088 deaths in hospitals in the US in 2002 [Citation68]. Assuming that 80% of these were due to catheter-induced infections [Citation16], that equates to 10 470 deaths. The population of the US is 4.98-times that of the UK, so the corresponding annual number of deaths in the UK is probably at least 2100.
In a postal survey to determine the incidence and morbidity of long-term catheterization in a typical National Health Service setting [Citation69], there were 506 referrals from a cohort of 457 patients over a 6-months period. From these referrals, 54 patients were selected for detailed study: 48% experienced catheter blockage, 37% reported urine by-passing the catheter and 30% noted haematuria.
Catheter-associated urinary tract infections not only place heavy demands on healthcare resources, but their treatment also raises profound concern regarding the development of antimicrobial resistance and they cause immense distress and social problems for the sufferers, their carers and their communities.
5.3. Economic implications
A rigorous analysis of economic costs of urinary retention, incontinence and catheterization is beyond the scope of this review. It may be helpful, however, to give some insight into the orders of magnitude involved, as follows:
In 2008, the world market for urinary continence care devices of all kinds (mainly catheters and pads) was estimated to be US$1.8 billion per year, growing at ∼7% per year; of this, Foley catheters accounted for ∼US$380 million in 2007 [Citation70]. The costs of the relevant devices are, however, small in relation to those of the clinical and societal consequences of incontinence and retention and its management by long-term catheterization. In the US in 2002, urinary tract infections were responsible for over 7 million physician visits; they accounted for more than 100 000 hospital admissions, mostly for pyelonephritis; and the direct and indirect costs associated with community-acquired urinary tract infections exceeded an estimated US$1.6 billion [Citation71]. In 1997, ∼15% of all community-prescribed antibiotics in Germany were dispensed for urinary tract infections, at an estimated cost of over US$1 billion [Citation72]. In 1991, it was estimated that an episode of nosocomial bacteriuria added US$500–1000 to the direct cost of acute-care hospitalization [Citation73].
The use of incontinence pads could be greatly reduced if there were a satisfactory alternative to the Foley catheter. In addition to the fact that the use of pads is difficult or impossible to conceal, thus tending to make sufferers socially reclusive, there is the further problem of progressive deterioration of the condition of their skin. Unless pads are changed so frequently that the skin is kept dry, it tends to become macerated, leading to pressure sores and, with repeated drenching, the problem becomes chronic and unmanageable. The cost of pads to the NHS in England increased from £77 million in 2006/2007 to £121 million in 2010/2011, so that their use has begun to be restricted. Until 2013, the allocation was based on clinical need. Now it is often based on financial considerations and, as a result, the allowance per patient can be as few as four disposable continence products in 24 h [Citation64]. This rationing perversely ignores the downstream costs of treating the resulting increase in the incidence of pressure ulcers, many of which are due to the use of pads and which, in the UK, was already between £1.4–£2.1 billion annually in 2004, accounting for 4% of total NHS expenditure [Citation74].
Clearly it is impossible to make a simple and accurate calculation of the financial cost attributable to catheter-associated urinary tract infections. According to hospital episode statistics, there were 281 296 finished consultant episodes of serious adverse events related to urinary tract infections in National Health Service hospitals (and private hospitals undertaking work for the NHS) in England and Wales in 2012–2013 [Citation75]. It is reasonable to assume that 80% of these episodes (225,036) were due to catheter-induced infections [Citation16]. Data for 2001 show that infected patients, on average, incurred UK hospital costs 2.9-times higher than uninfected patients, at that time equal to an additional £3154 per patient [Citation76]. Between 2001–2014, the UK consumer price index increased by a factor of 1.48 [Citation77]. Admittedly this may not be a very good index of healthcare cost inflation, but no better measure seems to be available. Thus, the additional UK National Health Service cost of each catheter-associated urinary tract infection must be in the order of £4600 so, by this calculation, the total annual cost of all episodes must be at least £1.0 billion.
Data for Scotland in 1999 [Citation78] gave an estimated £125 million as the additional annual cost of treating catheter-associated urinary tract infections. Scotland accounts for ∼8% of the UK population and financial inflation from 1999 to 2015 was ∼55%. This calculation indicates that the total current UK cost of catheter-associated urinary tract infections is ∼£2.5 billion per year.
Thus, it can be concluded that the annual total additional UK cost of catheter-associated urinary tract infections probably now lies somewhere between £1.0–£2.5 billion.
For the US, it was recently estimated that healthcare-acquired infections account for nearly US$45 billion per year in direct hospital costs [Citation79]. Assuming that 80% were due to catheter-induced infections [Citation16], the total annual cost must be ∼US$36 billion.
Prior to 1 October 2008, hospitals in the US were able to recover additional payment to compensate for the extra cost of treatment of catheter-associated urinary tract infections. Then the Medicare rule was changed and reimbursement for this was stopped [Citation80]. Whether or not this was justified, the rationale was that catheter-associated urinary tract infection could reasonably be prevented through the application of evidence-based guidelines. Whatever the other effects of this change in the rule have been, however, it is perhaps surprising that it does not seem yet to have been seen as an incentive to develop a better catheter.
Of course, the availability of a better catheter would not completely eliminate catheter-associated urinary tract infections but, even if only 50% of its potential could be achieved, the annual savings would be in the order of £500 million in the UK and US$18 billion in the US. Furthermore, over 1000 deaths would probably be avoided each year in the UK and over 5000 in the US.
6. Research agenda
It has rightly been said to be healthcare’s hidden scandal of neglect that the Foley catheter, a device originated in the 1930s and which is directly responsible for tremendous morbidity and significant mortality is still in routine long-term clinical use today [Citation81].
In order to meet this challenge, an alternative indwelling catheter system must be developed. Taking into account the data presented in this review, as well as other analyses [Citation82–84], the following research agenda can be proposed:
The catheter should be easy to insert and withdraw. This means that the catheter should be flexible and experience minimal friction with the urethra, possibly by the application of durable, lubricious, antibacterial and hydrophilic coating [Citation85].
The catheter should be retained within the bladder. The mechanism of retention is arguably the pivotal aspect of design development. The balloon of the Foley catheter provides a retention force between 9–41 N, depending on the balloon inflation volume [Citation86], which normally provides satisfactory retention, but it is associated with many problems (mainly incomplete urinary drainage, bladder and urethral damage), so a novel approach is essential.
The catheter should allow the bladder to fill at low pressure and to empty completely, mimicking the natural physiology and without damage to the urothelial lining of the bladder or the urethra. This “bioinspired” approach, based on the evidence of normal biological function, would surely minimize the occurrence of infection. For instance, an alternative to the balloon of the Foley catheter as a retention device should minimize the retained volume of urine. A catheter with numerous drainage eyes, extending from a suprapubic port to the urethral opening, could be retained suprapubically, with negligible volume within the bladder. A collapsible section in the catheter close to the end of the urethra or the suprapubic port could serve to restrict the rate of flow towards the end of drainage and, thus, to reduce the suction which otherwise could lead to pseudopolyps and other complications at the drainage eyes of the catheter. The catheter system should be able to minimize any increase in bladder pressure (such as that due to muscle spasm), to avoid damage to the kidneys. One possibility might be to provide an external reservoir to compensate for the reduction in the functional capacity of the bladder when it contracts during a spasm, with the urine flowing back into the bladder as it subsequently relaxes. Such a reservoir could also compensate for the reduction in the structural capacity of the bladder due to long-standing inflammation.
Catheter blockage is primarily the result of urinary infection and so the catheter should be resistant to encrustation by crystalline bacterial biofilm. Attempts to prevent catheter encrustation by impregnating the catheter surface with antimicrobial agents such as silver (see Section 3) have failed because of a lack of understanding of the encrustation process. Thus, it has been shown that, in patients infected with Proteus mirabilis, crystals generated in the alkaline urine rapidly cover the silver surface, protected from the underlying antibacterial agent [Citation87,Citation88]. The lesson here is clear: if antimicrobials are to be incorporated into catheters to prevent encrustation, they must diffuse from the catheter into the urine and, thus, prevent the bacteria from elevating the urinary pH. As a precautionary measure, a sensor might be used to predict imminent encrustation. Such a sensor already exists: strips of cellulose-acetate/bromothymol-blue polymer change their colour from yellow to dark blue when the pH of the urine increases due to the presence of Proteus mirabilis and this colour change has been shown reliably to occur some 12 days before catheter blockage [Citation89]. Moreover, the incidence and severity of catheter encrustation and blockage could be expected to be reduced if the normal periodic drainage of the bladder were to be restored.
There should be an effective method for the safe insertion of the catheter by the suprapubic route. Ultrasonic guidance is satisfactory, but only when used by a trained practitioner [Citation90], so this needs to be deskilled. An alternative is to construct a permanent continent suprapubic port by the Mitrofanoff [Citation91] procedure, which involves the transplantation of the vermiform appendix and major surgery. In this context, there has been an attempt to use a plastic gastrostomy button as an alternative port [Citation92]. Neither of these approaches is completely satisfactory; research into biomaterials with tissue-integrating surfaces or scaffolds must be worth exploring, aimed at developing a prosthetic suprapubic port suitable for intermittent or indwelling catheterization.
The catheter should have control mechanisms appropriate for all users, including those with loss of manual or cognitive abilities. A valve is needed to mimic the normal cyclical filling and emptying of the bladder. For users with manual dexterity and cognitive ability, this valve can be a simple tap, although a pinch valve is preferable because it eliminates the possibility of bacterial ingress. For people who cannot use a manual valve, an electrically-actuated valve is necessary, with pre-set timing for those for whom that is required: a prototype device already exists [Citation93].
The intellectual property in the design of the catheter should be protected, as this will be the catalyst for its commercial manufacture, without which it cannot be introduced into clinical practice. The performance of the catheter will need to be tested in clinical trials and it will need to be shown to comply with the relevant medical device regulations. Rigorous cost-benefit analysis will also be needed to generate the data to justify the inevitably higher cost than that of the Foley catheter.
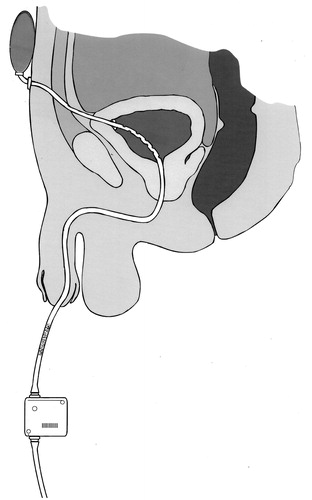
illustrates how some of these concepts, several of which are the subjects of patent applications [Citation94–96], could be incorporated in a catheter system designed to meet the specifications of the research agenda.
Figure 7. A catheter incorporating some of the concepts in the research agenda. The catheter is retained by wings which spring open after insertion through the suprapubic tract to the bladder: this traps less urine than the balloon of a Foley catheter and the catheter can be withdrawn transurethrally after cutting through it at the external suprapubic port. Multiple drainage eyes in the section of the catheter within the bladder minimize the risk of the formation of pseudopolyps, and this risk is further reduced by a collapsible section (shown stippled) of the catheter situated close to the external meatus of the urethra. The elastic reservoir at the suprapubic end of the catheter and strapped to the abdominal wall expands to accommodate urine from the bladder during spasmodic bladder contraction and returns it to the bladder when it relaxes after the spasm, thus minimizing the possibility of kidney damage. Periodic drainage of the bladder into a leg bag is actuated by a pinch valve beyond the collapsible section of the catheter, under manual or timed automatic control.
7. Conclusions
Whilst it is true that the huge clinical, social and economic costs of the use of the Foley catheter for long-term urinary drainage have come into sharper focus since the publication of Kunin’s seminal paper in 1988 [Citation38], it is regrettably also true that the scientific community, the relevant commercial companies and the regulatory authorities have largely failed to seek a solution. Perhaps the scientific community has not realized that there are significant interdisciplinary research challenges to be solved. The commercial companies probably see no reason to upset their business models with disruptive new technologies. The regulatory authorities apparently complacently neglect their responsibility to encourage innovation where existing devices are clearly inadequate.
This review ends with a research agenda for the achievement of safe long-term catheterization. The way forward is clear: now is the time for the research funders, the healthcare providers and the regulators to stimulate the scientific, engineering, commercial and clinical communities to meet the challenge.
Declaration of interest
R. C. L. Feneley is Emeritus Consultant Urologist at North Bristol NHS Foundation Trust; he and I. B. Hopley are Directors of Alternative Urological Catheter Systems Limited. P. N. T. Wells is Distinguished Research Professor at Cardiff University: he has no conflicts of interest to report.
References
- Hanafy, H.M., Saad, S.M., and Al-Ghorab, M.M., 1974, Ancient Egyptian Medicine. Contribution to urology. Urology, 4, 114–120
- Milne, J.S., 1907, Surgical instruments in Greek and Roman times (Oxford: Clarendon Press)
- Nacey, J., and Delahunt, B., 1993, The evolution and development of the urinary catheter. Australian and New Zealand Journal of Surgery, 63, 815–819
- Hanafy, H.M., Saad, S.M., El-Rafaie, M., and Al-Ghorab, M.M., 1976, Early Arabian medicine. Contribution to urology. Urology, 8, 63–67
- Herman, J.R., 1973, Urology: A view through the retrospectoscope (Maryland: Harper and Rowe)
- Hume, E.H., 1930, Medicine in China, old and new. Annals of Medical History, 2, 272–280
- Murphy, L.J.T., 1972, The history of urology (Springfield: C.C. Thomas)
- Paré, A., 1564, Dix livres de la chirurgie (Paris: Jean le Royer). First English translation by Johnson, T., 1634, The Collected Works of Ambroise Paré. (London: Th. Cotes and R. Young)
- Mattelaer, J.J., and Billiet, L., 1995, Catheters and sounds: The history of bladder catheterisation. Paraplegia, 33, 429–433
- Petit, J.J., 1790, Traité des maladies chirurgicales (Paris: M. Lesne)
- de Garengeot, J.C., 1731, Traité des operations de chirurgie (Paris: G. Cavalier)
- Thomas, G.J., 1933, History of Urology (Baltimore, MD: Williams and Wilkins)
- Reybard, J.F., 1855, Traité pratique des rétrécissements du canal de l’utère (Paris, Labè)
- Foley, F.E.B., 1937, A hemostatic bag catheter. Journal of Urology, 38, 137–139
- Mangelson, N.L., Kado, R.T., and Cockett, A.T.K., 1968, Silicone rubber uses in the lower urinary tract. Journal of Urology, 100, 573–577
- Maki, D.G., and Tambyah, P.A., 2001, Engineering out the risk for infection with urinary catheters. Emerging Infectious Diseases, 7, 342–347
- Greenspan, R.E., 2007, Medicine: Perspectives in history and art (Alexandria: Ponteverde Press)
- Zhang, D., Zhang, G., Hayden, M.S., Greenblatt, M.B., Bussey, C., Flavell, R.A., and Ghosh, H., 2014, A Toll-like receptor that prevents infection by uropathogenic bacteria. Science, 303, 1522–1526
- Lapides, J., Dionko, A.C., Sibler, S.J., and Lowe, B.S., 1972, Clean intermittent self-catheterisation in the treatment of urinary tract disease. Journal of Urology, 107, 458–461
- Webb, R.J., Lawson, A.L., and Neal, D.E., 1990, Clean intermittent self-catheterisation in 172 adults. British Journal of Urology, 65, 20–23
- Ouslander, J.G., Greengold, B., and Chen, S., 1987, External catheter use and urinary tract infections among incontinent male nursing home patients. Journal of the American Geriatrics Society, 35, 1063–1070
- Golji, H., 1981, Complications of external condom drainage. Paraplegia, 19, 189–197
- Armstrong, E.P., and Ferguson, T.A., 1988, Urinary incontinence: Healthcare resource consumption in Veterans Affairs medical centers. Veterans Health Systems Journal, October, 37–42
- Pel, J.J.M., and van Mastrigt, R., 2006, Development of the condom catheter method for non-invasive measurement of bladder pressure. Urodinamica, 16, 270–278
- Willette P.A., and Coffield, S., 2012, Current trends in the management of difficult urinary catheterizations. Western Journal of Emergency Medicine, 13, 472–478
- Ahluwalia, R.S., Johal, N., Kouriefs, C., Kooiman, G., Montgomery, B.S.I., and Plail, R.O., 2006, The surgical risk of suprapubic catheter insertion and long-term sequelae. Annals of the Royal College of Surgeons of England, 88, 210–213
- Harrison, S.C., Lawrence, W.T., Morley, R., Pearce, I., and Taylor, J., 2011, British Association of Urological Surgeons’ suprapubic catheter practice. BJU International, 107, 77–85
- McGill, S., 1982, Catheter management: It’s the size that’s important. Nursing Mirror, 154, 48–49
- Ruutu, M., Alfthan, O., Talja, M., and Andersson, L.C., 1985, Cytotoxicity of latex urinary catheters. British Journal of Urology, 57, 82–87
- Shenot, P., Rivas, D.A., Kalman, D.D., Staas, W.E., and Chancellor, M.B., 1994, Latex allergy manifested in urological surgery and care of adult spinal cord injured patients. Archives of Physical Medicine and Rehabilitation, 75, 1263–1265
- Stensballe, J., Looms, D., Nielsen, P.N., and Tvede, M., 2005, Hydrophilic-coated catheters for intermittent catheterisation reduce urethral micro trauma: A prospective, randomised, participant-blinded, crossover study of three different types of catheters. European Urology, 48, 978–983
- Lawrence, E.L., and Turner, I.G., 2006, Kink, flow and retention properties of urinary catheters part 1: Conventional Foley catheters. Journal of Materials Science: Materials in Medicine, 17, 147–152
- Stickler, D., Young, R., Jones, G., Sabbuba, N., and Morris, N., 2003, Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urological Research, 31, 306–311
- Johnson, J.R., Kuskowski, M.A., and Wilt, T.J., 2006, Systematic review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Annals of Internal Medicine, 144, 116–126
- Stickler, D.J., 2014, Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. Journal of Internal Medicine, 276, 120–129
- Pickard, R., Lam, T., Maclennan, G., Starr, K., Kilonzo, M., McPherson, G., Gillies, K., McDonlad, A., Walton, K., Buckley, B., Glazener, C., Boachie, C. Burr, J., Norrie, J., Vale, L., Grant, A., and N’dow, J., 2012, Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: Multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial). Health Technology Assessment, 16, 1–197
- Riley, D.K., Classen, D.C., Stevens, L.E., and Burke, J.P., 1995, A large randomized clinical trial on a silver-impregnated urinary catheter: Lack of efficacy and staphyllococal superinfection. American Journal of Medicine, 98, 349–356
- Kunin, C.M., 1988, Can we build a better urinary catheter? New England Journal of Medicine, 319, 365–366
- European Commission, 2010, Guidelines on Medical Devices: Serious Adverse Event Reporting. MEDDEV 2.7/3 (Brussels: Directorate General for Health and Consumers)
- Laver, C.H., 1917, An automatic bladder irrigator. Guy’s Hospital Gazette, 31, 71–74
- Munro, D., 1936, The activity of the urinary bladder as measured by a new and inexpensive cystometer. New England Journal of Medicine, 214, 617–624
- Riches, E.W., 1943, The methods and results of treatment in cases of paralysis of the bladder following spinal injury. British Journal of Surgery, 31, 135–146
- World Health Organisation, 2014, Antimicrobial Resistance Global Report on Surveillance (Geneva: WHO)
- Garcia, M.M., Gulati, S., Liepmann, D., Stackhouse, G.B., Greene, K., and Stoller, M.L., 2007, Traditional Foley drainage systems – do they drain the bladder? Journal of Urology, 177, 203–207
- Stickler, D.J., and Feneley, R.C.L., 2010, The encrustation and blockage of long-term indwelling bladder catheters: A way forward in prevention and control. Spinal Cord, 48, 784–790
- Stickler, D.J., and Morgan, S.D., 2006, Modulation of crystalline Proteus mirabilis biofilm development in urinary catheters. Journal of Medical Microbiology, 55, 489–494
- Khan, A., Housami, I., Melloti, R., Timoney, A., and Stickler, D., 2010, Strategy to control catheter encrustation with citrated drinks: A randomized crossover study. Journal of Urology, 183, 1390–1394
- Samson, G., and Cardenas, D.D., 2007, Neurogenic bladder in spinal cord injury. Physical Medicine and Rehabilitation Clinics of North America, 18, 255–274
- Kunin, C.M., 1989, Blockage of urinary catheters: Role of microorganisms and constituents of the urine on formation of encrustations. Journal of Clinical Epidemiology, 42, 835–842
- Williams, G.J., and Stickler, D.J., 2008, Effect of triclosan on the formation of crystalline biofilms by mixed communities of urinary tract pathogens on urinary catheters. Journal of Medical Microbiology, 57, 1135–1140
- Stickler, D.J., and Jones, G.L, 2008, Reduced susceptibility of Proteus mirabilis to triclosan. Antimicrobial Agents and Chemotherapy, 52, 991–994
- Feneley, R.C.L., Painter, D., Evans, A., and Stickler, D.J., 2002, Bladder catheterisation. British Journal of General Practice, 52, 500
- Glahn, B.E., 1988, Influence of drainage conditions on mucosal bladder damage by indwelling catheters. I. Pressure study. Scandinavian Journal of Urology and Nephrology, 22, 87–92
- Milles, G., 1965, Catheter-induced hemorrhagic pseudopolyps of the urinary bladder. Journal of the American Medical Association, 193, 968–969
- Russell, J.A., 2008, The current management of septic shock. Minerva Medica, 99, 431–458
- Kashefi, C., Messer, K., Barden, R., Sexton, C., and Parsons, J.K., 2008, Incidence and prevention of iatrogenic urethral injuries. Journal of Urology, 179, 2254–2258
- Parkin, J., Scanlan, J., Woolley, M., Grover, D., Evans, A., and Feneley, R.C., 2002, Urinary catheter “deflation cuff” formation: Clinical audit and in vitro analysis. BJU International, 90, 666–671
- Crisp, J.M., and Nacey, J.N., 1990, Foley catheter balloon fracture and the risk of free fragment formation. British Journal of Urology, 66, 500–502
- http://www.bjuinternational.com/bjui-blog/the-indwelling-foley-catheter-an-anachronism/
- Meddings, J., Rogers, M.A.M., Krein, S.L., Fakih, M.G., Olmsted, R.N., and Saint, S., 2014, Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: An integrative review. BMJ Quality and Safety, 23, 277–289
- Nicolle, L.E., 2014, Catheter associated urinary tract infections. Antimicrobial Resistance and Infection Control, 3, 23–29
- Lee, Y.Y., Tsay, W.L., Lou, M.F., and Dai, Y.T., 2007, The effectiveness of implementing a bladder ultrasound programme in neurosurgical units. Journal of Advanced Nursing, 57, 192–200
- Elsamra, S.E., Gordon, Z., and Ellsworth, P.I., 2011, The pitfalls of BladderScanTM PVR in evaluating bladder volume in adolescent females. Journal of Pediatric Urology, 7, 95–97
- All Party Parliamentary Group for Continence Care: Survey Report. England 2013 (http://www.appgcontinence.org.uk)
- Thom, D.H., Haan, N.M., and van den Eeden, S.K., 1997, Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age and Ageing, 26, 367–374
- Ouslander, J.G., Palmer, M.H., Rovner, B.W., and German, P.S., 1993, Urinary incontinence in nursing homes: Incidence, remission and associated factors. Journal of the American Geriatrics Society, 41, 1083–1089
- 2005, Report of the National Audit of Continence Care for Older People (65 years and above) in England, Wales and Northern Ireland (London: Royal College of Physicians)
- Klevens, R.M., Edwards, J.R., Richards, C.L, Horan, T.C., Gaynes, R.P., Pollock, D.A., and Cardo, D.M., 2007, Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Reports, 122, 160–166
- Kohler-Ockmore, J., and Feneley, R.C., 1996, Long-term catheterisation of the bladder: Prevalence and morbidity. British Journal of Urology, 77, 347–351
- 2009, Urinary Incontinence Devices Market: An Analysis (Vaishali: Koncept Analytics)
- Foxman, B., 2002, Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. American Journal of Medicine, 113 (Suppl 1A), 5S–13S
- Ruden, H., Gastmeier, P., Daschner, F.D., and Schumacher, M., 1997, Nosocomial and community acquired infections in Germany. Summary of the results of the First National Prevalence Study (NIDEP). Infection, 25, 199–202
- Patton, J.P., Nash, D.B., and Abrutyn, E., 1991, Urinary tract infection: Economic considerations. Medical Clinics of North America, 75, 495–513
- Bennett, G., Dealey, C., and Posnett, J., 2004, The cost of pressure ulcers in the UK. Age and Ageing, 33, 230–235
- Health & Social Care Information Centre, 2013 (http://www.hscic.gov.uk/catalogue/PUB12566)
- Plowman, R., Graves, N., Griffin, M.A.S., Roberts, J.A., Swan, A.V., Cookson, B., and Taylor, N., 2001, The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. Journal of Hospital Infection, 47, 198–209
- Office of National Statistics, 2015 (http://www.ons.gov.uk/ons/taxonomy/index.html?nscl-Economy)
- Catheter Associated Urinary Tract Infection (CAUTI) Surveillance. Health Protection Scotland, 2004. (http://www.hps.scot.nhs.uk/haiic/sshaip/publicationsdetail.aspx?id=30290)
- Kennedy, E.H., Greene, M.T., and Saint, S., 2013, Estimating the cost of catheter-associated urinary tract infection. Journal of Hospital Medicine, 8, 519–522
- Saint, S., Meddings, J.A., Calfee, D., Kowalsk,i C.P., and Krein, S.L., 2009, Catheter-associated urinary tract infection and the Medicare rule changes. Annals of Internal Medicine, 150, 877–884
- Reid, M., 2011, Spinal Column: My Performance Review (London: The Times)
- Dellimore, K.H., Heyler, A.R., and Franklin, S.E., 2013, A scoping review of important urinary catheter induced complications. Journal of Materials Science: Materials in Medicine, 24, 1825–1835
- Feneley, R.C.L., Kunin, C.M., and Stickler, D.J., 2011, An indwelling urinary catheter for the 21st century. BJU International, 109, 1746–1749
- Carr, H.A., 2000, A short history of the Foley catheter: From handmade instrument to infection-prevention device. Journal of Endourology, 14, 5–8
- Ahmed, N., and Al-Lamee, K., 2008, A hydrophilic technology for intermittent urinary catheters. Medical Device Technology, 19, 17–19
- Wu, A.K., Blaschko, S.D., Garcia, M., McAninch, J.W., and Aaronson, D.S., 2012, Safer urethral catheters: How study of catheter balloon pressure and force can aid design. BJU International, 109, 1110–1114
- Stickler, D.J., and Morgan, S.D., 2008, Observations on the development of the crystalline bacterial biofilms that encrust and block Foley catheters. Journal of Hospital Infection, 69, 350–360
- Morgan, S.D., Rigby, D., and Stickler, D.J., 2009, A study of the structure of the crystalline biofilms encrust and block silver Foley catheters. Urological Research, 37, 89–93
- Stickler, D.J., Jones, S.M., Adusei, G.O., Waters, M.G., Cloete, J., Mathur, S., and Feneley, R.C., 2006, A clinical assessment of the performance of a sensor to detect crystalline biofilm formation on indwelling bladder catheters, BJU International, 98, 1244–1249
- Jacob, P., Rai, B.P., and Todd, A.W., 2012, Suprapubic catheter insertion using an ultrasound-guided technique and literature review. BJU International, 110, 779–784
- Mitrofanoff, P., 1980, Trans-appendicular continent cystostomy in the management of the neurogenic bladder (in French). Chirurgie Pédiatrique, 21, 297–305
- Hitchcock, R.J., and Sadiq, M.J., 2007, Button vesicostomy: A continent urinary stoma. Journal of Pediatric Urology, 3, 1004–1008
- Lee, S.M., Short, T.D., and Unsworth, A., 2007, Design and development of a novel automatic valve system for long-term catheterized urinary incontinence patients. Proceedings of the Institution of Mechanical Engineers [H], 221, 665–676
- PCT/GB2010/001523; Europe: 10807469.1; USA: 13/391866
- PCT/GB2012/000132; Europe: 12704522.7; USA; 13/985771
- UK: 1314140.3; PCT/2014/052277