Abstract
Although daily rhythms regulate multiple aspects of human physiology, rhythmic control of the metabolome remains poorly understood. The primary objective of this proof-of-concept study was identification of metabolites in human plasma that exhibit significant 24-h variation. This was assessed via an untargeted metabolomic approach using liquid chromatography–mass spectrometry (LC-MS). Eight lean, healthy, and unmedicated men, mean age 53.6 (SD ± 6.0) yrs, maintained a fixed sleep/wake schedule and dietary regime for 1 wk at home prior to an adaptation night and followed by a 25-h experimental session in the laboratory where the light/dark cycle, sleep/wake, posture, and calorific intake were strictly controlled. Plasma samples from each individual at selected time points were prepared using liquid-phase extraction followed by reverse-phase LC coupled to quadrupole time-of-flight MS analysis in positive ionization mode. Time-of-day variation in the metabolites was screened for using orthogonal partial least square discrimination between selected time points of 10:00 vs. 22:00 h, 16:00 vs. 04:00 h, and 07:00 (d 1) vs. 16:00 h, as well as repeated-measures analysis of variance with time as an independent variable. Subsequently, cosinor analysis was performed on all the sampled time points across the 24-h day to assess for significant daily variation. In this study, analytical variability, assessed using known internal standards, was low with coefficients of variation <10%. A total of 1069 metabolite features were detected and 203 (19%) showed significant time-of-day variation. Of these, 34 metabolites were identified using a combination of accurate mass, tandem MS, and online database searches. These metabolites include corticosteroids, bilirubin, amino acids, acylcarnitines, and phospholipids; of note, the magnitude of the 24-h variation of these identified metabolites was large, with the mean ratio of oscillation range over MESOR (24-h time series mean) of 65% (95% confidence interval [CI]: 49–81%). Importantly, several of these human plasma metabolites, including specific acylcarnitines and phospholipids, were hitherto not known to be 24-h variant. These findings represent an important baseline and will be useful in guiding the design and interpretation of future metabolite-based studies. (Author correspondence: [email protected] or [email protected])
INTRODUCTION
Metabolomics is the study of small molecule (<1 kDa) metabolic profiles in biological systems, and complements genomic and proteomic approaches in providing global views of biological processes. Metabolic profiles capture endogenous and exogenous influences on a living organism and may provide better representation of its functional phenotype than changes in DNA, RNA, and proteins (Allen et al., Citation2003; Nicholson et al., Citation2002). Hence, metabolic perturbations caused by such disparate factors as genetic changes, microbes, diseases, food, and therapeutic interventions may be investigated using a metabolomic approach (Allen et al., Citation2003; Nicholson et al., Citation2002).
Recently published studies have identified clear and pervasive circadian influence on the murine hepatic metabolome (Eckel-Mahan et al., Citation2012; Fustin et al., Citation2012). Plasma metabolites in a mouse model that vary significantly with time-of-day have crucially been identified in a landmark study; 14 oscillating metabolites were identified by liquid chromatography–mass spectrometry (LC-MS) and 28 by capillary electrophoresis–mass spectrometry (Minami et al., Citation2009). Direct translation of these animal data to humans, however, cannot be made. In addition to the obvious problem of comparing diurnal and nocturnal species, the timing and amount of feeding, activity, rest, sleep, and posture were not controlled in these animal experiments.
Although daily rhythms regulate multiple aspects of human physiology, rhythmic control of the plasma metabolome remains poorly understood. Previous studies have investigated time-of-day variation in the human metabolome by comparing metabolomic profiles of morning with early evening urine samples (Slupsky et al., Citation2007; Walsh et al., Citation2006). Although both studies identified significant changes in metabolite levels, such as increased creatinine and dimethylamine in the morning samples, comparison of samples collected at only two time points does not provide comprehensive overview of variation across the 24-h day. Furthermore, these studies did not control for sleep, activity, medication, alcohol/caffeine intake, or environmental lighting, and thus, the results obtained are extremely unlikely to show nonconfounded time-of-day variation. To overcome this, a recent study employed a targeted platform to study the human plasma metabolome under controlled laboratory conditions (Dallmann et al., Citation2012). The analytical platform, however, was limited to 281 metabolites, and plasma samples from all subjects were pooled at each time point, precluding analyses of intersubject variability and intrasubject daily variation and potentially reducing the sensitivity of this methodology. The current study circumvented these limitations by the use of a global, untargeted metabolomic approach in the analysis of individual plasma samples collected from healthy human volunteers at different time points. The aim of this proof-of-concept study was characterization of 24-h variation of the plasma metabolome in human subjects maintained under highly controlled conditions of food, posture, light/dark cycle, sleep/wake schedule, and prior exposure to pharmacologic agents. The technological platform with LC-MS used in this study has been previously validated by our group with over a thousand metabolites in plasma being reproducibly detected (Pandher et al., Citation2009, Citation2012).
MATERIALS AND METHODS
All aspects of the study were conducted in accordance with the Declaration of Helsinki and conformed to international ethical standards (Portaluppi et al., Citation2010). A favorable ethical opinion was obtained from the Surrey Research Ethics Committee and the University of Surrey Ethics Committee. Written informed consent was obtained from all participants.
Eligibility for the study was determined via self-completed questionnaires, including General Health Questionnaire, General Sleep Questionnaire, Horne-Östberg Questionnaire, Pittsburgh Sleep Quality Index, Beck Depression Inventory, and Epworth Sleepiness Scale, to assess general health, sleep patterns, and diurnal preference. Full details of the screening process have been previously presented (Otway et al., Citation2011). To be included, subjects needed to report a regular sleep schedule of between 6 and 8 h in duration and not be extreme morning or evening chronotype according to the Horne-Östberg Questionnaire (Horne & Östberg, Citation1976). Subjects were excluded if they were taking regular medication or food supplements known to influence metabolism, inflammatory markers, endothelial markers, sleep, or the circadian system, or if they consumed more than four caffeinated beverages per day. Subjects with a history of any of the following were also excluded: (a) circadian or sleep disorder; (b) metabolic, cardiovascular, or chronic infectious/inflammatory disease; (c) psychiatric or neurological disease; and (d) drug and alcohol abuse.
Eight lean, male volunteers were recruited with a mean age of 53.6 (SD ±6.0) yrs, mean body mass index (BMI) of 23.2 kg/m2 (SD ±1.4), and fasting glucose of 4.2 mmol/L (SD ±.7). One participant was a smoker, but refrained from smoking for 1 wk prior to study. No participant had undertaken shiftwork within 5 yrs or crossed any time zones within 1 mo of the study. For 1 wk prior to the laboratory study, volunteers were required to maintain scheduled daily meal times (monitored by food diaries) and a fixed sleep/wake schedule (23:00–07:00 h), confirmed via wrist actigraphy (Actiwatch-L; Cambridge Neurotechnology, Cambridge, UK), sleep diaries, and calling a time-stamped voicemail (Otway et al., Citation2011). Participants also abstained from eating fatty or sugary foods and drinking alcohol or caffeine throughout this baseline week. For the final 3 d of this week, participants were provided with meals of specific nutritional content: the daily calorific content was 1.5-fold the basal metabolic rate (estimated in calories = 11.5 × body weight [kg] + 873), with ∼35% of energy derived from fat (Schofield, Citation1985).
In-laboratory Session
The in-laboratory session was conducted at the Surrey Clinical Research Centre. Following an adaptation night in the laboratory, subjects were woken at 06:30 h and commenced a 25-h experimental session throughout which they maintained a semi-recumbent posture to minimize the impact of exogenous factors on the measured parameters. Subjects remained awake in normal room lighting (range 440–825 lux in the direction of gaze) between 06:30 and 22:30 h and were allowed to sleep between 22:30 and 06:30 h in 0 lux. During the waking period, participants were provided with hourly nutritional drinks (Fortisip; Nutricia, Schiphol, The Netherlands) and were allowed to drink water ad libitum. The hourly consumption of this drink met the protein, carbohydrate, fat, and fiber requirements of each participant. Daily energy intake was 1.1-fold basal metabolic rate spread equally over the waking hours. Throughout the protocol, including overnight, blood samples were collected via an indwelling cannula by a qualified person, and attempts were made to minimize any disruption of the participants' sleep. Blood samples were collected at selected time points (.5 mL/time point for metabolomic analysis) into lithium heparin tubes, and the plasma fraction was separated by centrifugation (3000 × g, 10 min, 4°C) and stored at −80°C.
Reagents and Solutions
Water (LC-MS grade), acetonitrile (LC-MS grade), and formic acid (Aristar grade) were purchased from Fisher Scientific (Loughborough, UK), and leucine enkephalin was purchased from Sigma (Poole, UK). External standards, creatine (CAS number: 57-00-1), and colchicine (CAS number: 64-86-8), were purchased from Sigma.
Plasma Preparation
Samples from selected time points (07:00, 10:00, 16:00, 22:00, 04:00, and 07:00 h [d 2]) were extracted by mixing 1 volume of heparinized plasma with 4 volumes of methanol/ethanol 1:1, followed by centrifugation at 18 000 × g for 15 min at 4°C.
Assessment of Analytical Variability
Pooled plasma from the eight human subjects from all the sampled time points (i.e., 48 samples) served as quality controls and was analyzed throughout the experimental batch to continuously monitor the analytical variability of the system. These quality-control samples (1/10 injections) were spiked with 1 mM colchicine and creatine. Variability of these spiked compounds and endogenous metabolites, including carnitine, phenylalanine, and lysophosphatidylcholine (lysoPC(16:0)), were evaluated.
LC-MS
Experiments were carried out on two LC-MS systems, namely Acquity UPLC coupled to QTOF Premier mass spectrometer (Waters Corporation, Manchester, UK) and Agilent 1290 Series UPLC connected to a hybrid quadrupole-time-of-flight Agilent 6510 mass spectrometer (Agilent, Waldbronn, Germany); the first system was used for full-scan analysis and the second system for MS/MS. The robustness, reproducibility, and cross-platform validation of the two systems in studying the exo-metabolome, including plasma, have been previously published (Pandher et al., 2009, 2012). To minimize systematic analytical drift from use of large sample numbers and as the analytical reproducibility of the system is high, one analytical replicate from each individual per time point was used. An electrospray ionization source in positive mode was used for both LC-MS setups in this proof-of-concept study, as the ions detected in the positive mode are known to represent a large proportion of the exo-metabolome in our analytical system (Pandher et al., Citation2009).
Briefly, chromatographic separation was performed on a Waters Acquity HSS T3 C18 (100 × 2.1 mm, internal diameter [I.D.] 1.8 µm) column. Mobile phase A was LC-MS-grade water containing .1% formic acid and mobile phase B was LC-MS-grade acetonitrile containing .1% formic acid. The column and the autosampler were maintained at a temperature of 50°C and 4°C, respectively. A 13-min linear gradient elution was performed as follows: 100% mobile phase A for the first .5 min, changing to 100% B over 7.5 min, holding at 100% B up to 9.5 min, and finally back to 100% A at 10 min and holding for 3 min. The flow rate was .6 mL/min, with an injection volume of 10 µL. The MS instrument and data acquisition parameters were as previously described (Pandher et al., Citation2009, Citation2012).
Data Handling and Statistical Considerations
Raw data were detected, aligned, and processed using MarkerLynx application manager software (version 4.1; Waters, Milford, MA, USA), with parameters documented previously (Pandher et al., Citation2009). Each metabolite feature was characterized by a unique combination of mass/charge ratio and retention time. The data matrix obtained was subsequently subjected to multivariate statistical analysis using (i) SIMCA-P v11.0 software (Umetrics AB, Umeå, Sweden): metabolite features that were differentially expressed in one or more of three chosen sets of selected time points—10:00 vs. 22:00 h, 16:00 h vs. 04:00 h, and 07:00 h (d 1) vs. 16:00 h—were identified using orthogonal partial least squares-discriminant analysis (OPLS-DA) with a low threshold of |p(corr)| > .5 on the OPLS-DA S-plot (Wiklund et al., Citation2008); and (ii) repeated-measures analysis of variance (ANOVA) with time as an independent variable; statistical significance was deemed to be achieved at p < .05. Extracted ion chromatograms (EICs) of the selected metabolite features were then generated using QuanLynx application manager software (version 4.1; Waters). Finally, cosinor analysis using the mean peak height of EICs of all metabolite features of interest at each time point by the method of least squares (period of 24 h) was carried out to derive estimates by the cosine curve approximation of MESOR (24-h time series mean), amplitude (one-half peak-to-trough variation), acrophase (peak) time, and p value for test of the null hypothesis that the amplitude of the fitted curve was 0; (Nelson et al., Citation1979); rhythm detection was considered statistically significant when p < .05 for the zero-amplitude test.
Metabolite Identification
The accurate mass and tandem MS fragmentation pattern of each metabolite feature of interest was ascertained and identification performed by database searching (including Human Metabolome Database, Lipid maps, and Metlin) and/or comparison with pure commercial standards. MS/MS was performed on the Agilent system with a default iso-width (width half-maximum of the quadrupole mass bandpass used during MS/MS precursor isolation) of 4 m/z using a fixed collision energy of 15V and data acquired in the range of 50 to 800 Da.
RESULTS
Analytical Reproducibility
Within the pooled plasma quality-control samples, coefficients of variation for endogenous metabolites (carnitine, phenylalanine, and lysoPC(16:0)) were 1.7%, 3.0%, and 8%, respectively, whereas that of spiked exogenous compounds (creatine and colchicine) were 3.6% and 7.7%, respectively.
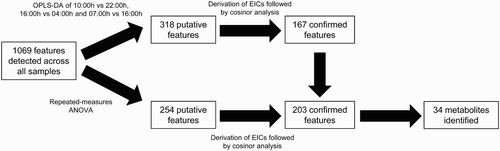
Details of Workflow
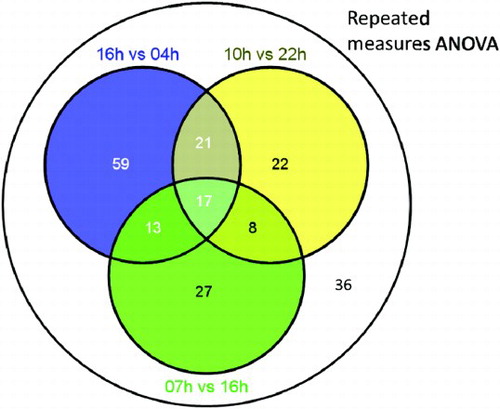
summarizes our data analysis workflow. In the present study, a total of 1069 metabolite features were detected across all analyzed plasma samples. Of these, 318 features passed the OPLS-DA filter using three pairwise comparisons of 10:00 vs. 22:00 h, 16:00 vs. 04:00 h, and 07:00 vs. 16:00 h. Subsequently, 167 features were confirmed to be significantly 24-h variant using cosinor analysis of EIC data (p < .05). In parallel, using repeated-measures ANOVA, 254 putative features were detected, and 203 were confirmed to exhibit 24-h variation. The 203 confirmed features detected by repeated-measures ANOVA represented the sum total of all the temporally variant features detected in this study (19% of all detected features in this study) and included all 167 features identified by OPLS-DA and 36 features additionally identified by repeated-measures ANOVA.
Of a total of 203 24-h variant features, the levels of 110 (54%) were significantly different between 16:00 and 04:00 h, whereas those of 68 (33%) and 65 (32%) were significantly different between 10:00 and 22:00 h, and 07:00 and 16:00 h, respectively. Repeated-measures ANOVA detected 36 features unique to those obtained from the stated paired comparisons. These results are summarized in Supplementary and Supplementary . Using a combination of accurate mass, tandem MS, and online database searches, the identities of 34 metabolites were determined from the 203 rhythmic features. Fragmentation patterns and properties of these compounds on our LC-MS system are summarized in .
TABLE 1. LC-MS characteristics of identified, 24-h variant plasma metabolites and key parameters characterizing temporal variation
Variation of Human Plasma Metabolite Levels Across Time-of-Day
Metabolites showing significant 24-h variation were from a variety of chemical classes and included acylcarnitines, lysophospholipids, bilirubin, corticosteroids, and amino acids. For these identified compounds, the mean ratio of oscillation range relative to the MESOR was 65% (95% confidence interval [CI]: 49–81%). Biological variability was consistently greater than analytical variability; the mean analytical coefficient of variation of these ions was 8% (95% CI: 6–10%).
The fitted peak times of the identified 24-h varying plasma metabolites were spread across the day, but appeared to be clustered around early morning, afternoon, and evening (Table 1). Levels of long-chain unsaturated acylcarnitines (C14:1, C14:2, and C18:1) peaked before the long-chain saturated acylcarnitines (C10, C12, C14, C16, and C18) in the morning, whereas the acrophases of short-chain acylcarnitines were observed at radically different times across the day (C2 at 4.9 h, C6 at 5.4 h, C3 at 14.3 h, and C4 at 17.7 h). Bilirubin and cortisol peaked after the start of the light phase, whereas the levels of detected amino acids, such as methionine, tyrosine, proline, lysine, phenylalanine, and leucine, were highest from mid- to late afternoon. By contrast, levels of carnitine, alanine, arginine, tryptophan, and valine (Supplementary ) did not vary significantly over the 24 h. Of the detected phospholipids, lysophosphatidylethanolamines (lysoPEs) peaked in the late afternoon and early evening, followed by the phosphatidylcholines (PCs), which peaked later in the evening. In contrast, two other phosphocholines (lysoPC(16:0) and lysoPC(18:1)) (Supplementary ) detected in our analytical system had levels that did not change significantly over the day.
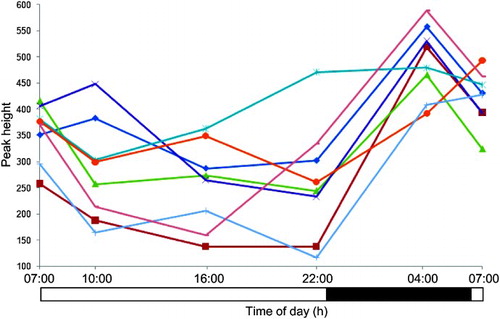
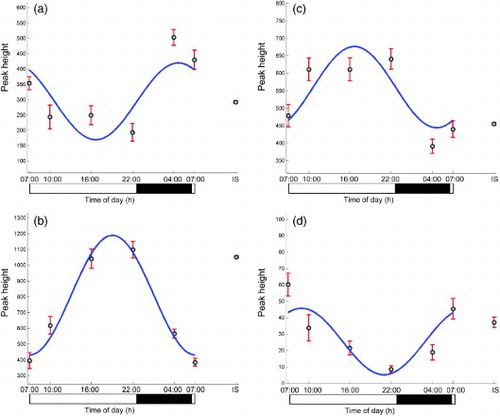
The peak height versus time profiles of four selected metabolites are presented in , whereas additionally shows the time profile of one of the metabolites of interest, acetylcarnitine, in each of the eight subjects.
FIGURE 2. Time profiles of four plasma metabolites with different acrophases: (a) acetylcarnitine, (b) LysoPE(18:1), (c) proline, and (d) cortisol. On the horizontal axis, black bar indicates lights-off (0 lux) and white bar lights-on (440–825 lux). Internal standard (IS) shows the analytical variation of each ion in the pooled, replicate samples analyzed throughout the LC-MS run.
DISCUSSION
In this study, an untargeted, global metabolomic approach was employed to discover novel 24-h rhythmic metabolites in human plasma obtained under highly controlled conditions. In this context, a UPLC-QTOF MS platform was chosen for its high reproducibility, sensitivity, and mass accuracy. It is stressed that rigorous conditions of sample collection are necessary, as the plasma metabolome is known to be exquisitely sensitive to a broad range of intrinsic and extrinsic factors (Lawton et al., Citation2008; Lewis et al., Citation2010; Psychogios et al., Citation2011). A panel of 24-h rhythmic metabolites from a range of chemical classes were identified, including bilirubin, corticosteroids, amino acids, acylcarnitines, and phospholipids. Nineteen percent of the metabolite features detected using our analytical platform exhibited significant daily variation, with the mean oscillation range over MESOR ratio of 65% (95% CI: 49–81%) in the identified metabolites, pointing to the presence of clinically significant time-of-day biological variation.
In order to identify daily rhythms in metabolites, two sets of time points 12-h apart were selected for comparison, the rationale being they have a periodicity of ∼24 h and typically oscillate in a manner that approximates a cosine curve with the peak and nadir occurring ∼12 h out of phase. To identify such rhythms in metabolites, sets of time points 9–12 h apart were selected for comparison to provide a good probability of detecting something that approximates to the maximum temporal difference. Repeated-measures ANOVA was additionally used to capitalize on the paired nature of the data, i.e., plasma was collected from eight individuals across time. This latter method was the most sensitive and yielded 36 features in addition to OPLS-DA comparisons (Supplementary ). Not only did this validate results already obtained by OPLS-DA, but it also demonstrated the effect of intersubject variability as well as the relatively higher sensitivity of this approach (Supplementary ). To illustrate this point, the 24-h temporal profile of a metabolite feature is presented in Supplementary , which highlights the significant intersubject variation in this ion compared to intrasubject time-of-day changes. In principle, other multivariate tools, such as the multilevel partial least square projection to latent structures (PLS), could be used instead of OPLS-DA for each pairwise comparison in the screening step, as it offers greater statistical power (Westerhuis et al., Citation2010). In this study, multilevel PLS was not carried out; instead, repeated-measures ANOVA was performed, making use of all data across all time points.
Our data extend previous findings that an unbiased metabolomic platform may be used to identify 24-h variant metabolites (Dallmann et al., Citation2012; Eckel-Mahan et al., Citation2012; Fustin et al., Citation2012; Minami et al., Citation2009; Slupsky et al., Citation2007; Walsh et al., Citation2006); some of the 24-h variant plasma metabolites detected in this study are already known to show such variation, including bilirubin, cortisol, and several amino acids (Eriksson et al., Citation1989; Feigin et al., Citation1967, Citation1968; Larsson et al., Citation2009; Selmaoui & Touitou, Citation2003; Wurtman et al., Citation1968), and this provides further validation of this approach. It is also noteworthy that numerous metabolites linked to major metabolic pathways were identified in this study; the 24-h variant nature of these metabolites has not been previously demonstrated.
Rhythms in human plasma levels of amino acids have been previously reported (Eriksson et al., Citation1989; Feigin et al., Citation1967, Citation1968; Wurtman et al., Citation1968). Typically, maximal concentrations were observed in the afternoon/evening, and minimal concentrations in the early hours of the morning before waking. Our results are in keeping with these findings: of the detected amino acids, lysine, proline, leucine, methionine, phenylalanine, and tyrosine exhibited significant day/night variation with similar peak/trough temporal changes. The mechanisms regulating this 24-h rhythmicity remain obscure, and they may be related to the periodicity of many other metabolic processes (Eckel-Mahan et al., Citation2012; Feigin et al., Citation1971). For instance, the tricarboxylic acid cycle, gluconeogenesis, and lipogenesis utilize amino acid carbon backbones and may be important in this context.
The present study identified numerous plasma acylcarnitines that demonstrated significant time-of-day variation; only a subset of these has thus far been reported (Dallmann et al., Citation2012). Acylcarnitines are key intermediates in the β-oxidation of fatty acids in mitochondria (Kompare & Rizzo, Citation2008), and abnormal levels of these metabolites have been linked to errors of metabolism involving fatty acid oxidation and carnitine cycle (Pasquali et al., Citation2006). Costa et al. (Citation1999) reported preferential increase in levels of specific mono-unsaturated acylcarnitines in fasting individuals, who generally display increased levels of blood unesterified fatty acids and hepatic β-oxidation. Fasting alone, however, is unlikely to account for the changes observed in the current study, as levels of many of these metabolites peaked and started to decrease in the morning before lights were switched on and feeding of hourly caloric drinks recommenced. Critical components of the biological system regulating the levels of acylcarnitines also demonstrate 24-h variation. For instance, using gene expression profiling data of liver enzymes across the time-of-day in a mouse model, mRNA levels of key transporters of long-chain acylcarnitines, including carnitine palmitoyltransferase (CPT) 1a and 2, were shown to exhibit clear day/night oscillation (Hughes et al., Citation2009).
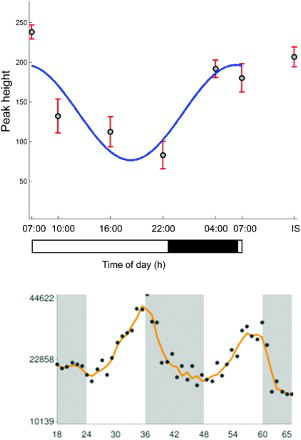
It is interesting to note that the time-of-day pattern of variation differs between short-chain, unsaturated long-chain, and saturated long-chain acylcarnitines. Levels of long-chain unsaturated acylcarnitines (C18:1, C14:1, and C14:2) peaked early in the morning before the long-chain saturated acylcarnitines (C10, C12, C14, C16, and C18), whereas the acrophases of short-chain acylcarnitines were observed at radically different times, i.e., C2 at 4.9 h, C6 at 5.4 h, C3 at 14.3 h, and C4 at 17.7 h. Abnormalities in particular enzyme and membrane transporter systems have already been associated with pathognomonic changes in acylcarnitine subtypes in well-defined subtypes of inborn errors of metabolism (Santra & Hendriksz Citation2010). Hence, it is plausible that short- and long-chain acyl dehydrogenases and transporters are regulated differently across time. Indeed, preliminary evidence suggests the presence of such a specific regulatory process. Whereas mRNA levels of CPT1a are rhythmic with an acrophase just before lights-off in the mouse—end of the inactivity span, levels of long-chain acylcarnitines transported by this mitochondrial membrane enzyme peak at the end of the dark phase in human subjects—also the end of inactivity span (selected profiles are juxtaposed in time relative to the activity/rest rhythm of the respective species in ). Given that humans and mice are diurnal and nocturnal species, respectively, agreement of these patterns of temporal change suggest that (CPT) 1a may be responsible for the 24-h variation observed in long-chain acylcarnitines. By contrast, expression profiles of (CPT) 1b and (CPT) 1c did not show significant time-of-day difference in the mouse, lending additional support for the hypothesis that (CPT) 1a may be the specific rhythmic driver of long-chain acylcarnitines (Hughes et al., Citation2009). It is important to point out that these cross-species associations, although interesting, are tentative and require further biological validation.
FIGURE 4. (Top panel) The temporal profile of palmitoylcarnitine in human plasma. (Bottom panel) CPT1a gene expression in a mouse model from an independent study (Hughes et al., Citation2009). Black/gray bars indicate lights-off and the white bar lights-on.
In this study, acrophases of specific C16, C18, and C20 lysoPCs and lysoPEs clustered in the afternoon and evening; no clear exogenous factor related to this could be identified, including food consumption. Two other endogenously expressed plasma phospholipids were assessed and showed no significant time-of-day change, suggesting possible specificity of the rhythmic driver for particular subtypes of lysophospholipids. Of note, temporal variation in plasma lysophospholipid levels has also been detected in mouse plasma (Minami et al., Citation2009). Enzyme systems currently understood to be mainly responsible for production of these metabolites are the lecithin:cholesterolacyltransferase (Lcat), secreted phospholipase A2 (Pla2), and endothelial lipase (Lipg) systems (Yamamoto et al., Citation2011); in a mouse model, Lcat, Pla2g12a, and Lipg are rhythmically expressed in the liver (Hughes et al., Citation2009).
The current results will be of use in a number of areas, for example, in the evaluation of pharmacodynamic biomarkers of new drug therapies (Sarker & Workman, Citation2007). Evaluation of pharmacodynamic biomarkers is made by drawing intrasubject comparisons across time with the patient pre-dose used as a control. This approach, however, may be confounded by the patient's internal circadian and homeostatic systems and external influences, such as dietary intake. The approach taken in the current study typifies a way to validate such pharmacodynamic studies by defining metabolites that are rhythmic and those that are not.
The findings of our study have implications on the use of plasma metabolites in clinical testing. At least 19% of the metabolite features in our study exhibited significant 24-h variation, with the mean oscillation range over MESOR ratio of 65% (95% CI: 49–81%) in the identified metabolites. To improve the predictive utility of clinical biomarkers used in diagnosis, prognosis, and follow-up, determination of 24-h variability should become part of the clinical validation process, especially in the ascertainment of the degree of 24-h variation relative to the factor(s) in question.
A limitation of the present study is that although a global, untargeted approach was adopted, only a proportion of the entire plasma metabolome was monitored using our analytical method. Despite this, the primary objective of identifying and characterizing novel 24-h variant plasma metabolites was met in this proof-of-concept study. Indeed, the present study identified all the 24-h variant metabolites detected by LC-MS in positive ionization mode in the Dallmann et al. (Citation2012) study that was conducted under “constant routine” conditions of constant dim lighting, continuous hourly food supplement, and no sleep, thereby providing external validation of their data. Significantly, additional 24-h variant plasma metabolites were identified in the present study, suggesting that these metabolites may be affected by lighting, sleep, and/or feeding condition. Alternatively, as the samples in the Dallmann et al. (Citation2012) study were pooled for each studied time point, the nondetection of these additional metabolites could be due to lower sensitivity of their approach. Although only eight subjects were studied with six time points across a 24-h day, multiple factors that could confound the rhythms were strictly controlled for. Specifically, study subjects were lean, healthy, and unmedicated and who maintained a fixed sleep/wake schedule and dietary regime for 1 wk at home prior to an adaptation night, followed by a 25-h session in the laboratory when the light/dark and sleep/wake cycle, posture, and calorific intake were rigorously controlled. In this context, it would be of interest to disentangle the impact of food, sleep, and darkness, and prospective clinical studies are ongoing to address this. In addition, the study participants were all middle-aged men, and the applicability of these findings to females and other age groups is unclear.
In summary, we have successfully applied the use of an untargeted metabolomic approach to identify and characterize 24-h variant metabolites from a range of chemical classes. These findings represent an important baseline and will be useful in guiding the proper design and interpretation of future metabolite-based studies.
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was funded by Diabetes-UK (grant 08/0003607), Biotechnology and Biological Sciences Research Council (grants BB/D526853/1 and BB/I019405/1) and Cancer Research UK (grants C309/A8274 and C309/A2187). J.E.A. receives funding support from the Wellcome Trust (grant 090952/Z/09/Z) and D.J.S. is a Royal Society Wolfson Research Merit Award holder. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- Allen J, Davey HM, Broadhurst D, Heald JK, Rowland JJ, Oliver SG, Kell DB. (2003). High-throughput classification of yeast mutants for functional genomics using metabolic footprinting. Nat. Biotechnol. 21:692–696.
- Costa CC, de Almeida IT, Jakobs C, Poll-The BT, Duran M. (1999). Dynamic changes of plasma acylcarnitine levels induced by fasting and sunflower oil challenge test in children. Pediatr. Res. 46:440–444.
- Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA. (2012). The human circadian metabolome. Proc. Natl. Acad. Sci. U. S. A. 109:2625–2629.
- Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. (2012). Coordination of the transcriptome and metabolome by the circadian clock. Proc. Natl. Acad. Sci. U. S. A. 109:5541–5546.
- Eriksson T, Voog L, Walinder J, Eriksson TE. (1989). Diurnal rhythm in absolute and relative concentrations of large neutral amino acids in human plasma. J. Psychiatr. Res. 23:241–249.
- Feigin RD, Klainer AS, Beisel WR. (1967). Circadian periodicity of blood amino-acids in adult men. Nature 215:512–514.
- Feigin RD, Klainer AS, Beisel WR. (1968). Factors affecting circadian periodicity of blood amino acids in man. Metabolism 17:764–775.
- Feigin RD, Beisel WR, Wannemacher RW (1971). Rhythmicity of plasma amino acids and relation to dietary intake. Am. J. Clin. Nutr. 24:329–341.
- Fustin M, Doi M, Yamada H, Komatsu R, Shimba S, Okamura H. (2012). Rhythmic nucleotide synthesis in the liver: temporal segregation of metabolites. Cell Reports 1:341–349.
- Horne JA, Östberg O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol 4:97–110.
- Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda S, Hogenesch JB. (2009). Harmonics of circadian gene transcription in mammals. PLoS Genet. 5:e1000442.
- Kompare M, Rizzo WB. (2008). Mitochondrial fatty acid oxidation disorders. Semin. Pediatr. Neurol. 15:140–149.
- Larsson A, Hassan M, Ridefelt P, Axelsson J. (2009). Circadian variability of bilirubin in healthy men during normal sleep and after an acute shift of sleep. Chronobiol. Int. 26:1613–1621.
- Lawton KA, Berger A, Mitchell M, Milgram KE, Evans AM, Guo L, Hanson RW, Kalhan SC, Ryals JA, Milburn MV. (2008). Analysis of the adult human plasma metabolome. Pharmacogenomics 9:383–397.
- Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, Souza A, Cheng S, McCabe EL, Yang E, Shi X, Deo R, Roth FP, Asnani A, Rhee EP, Systrom DM, Semigran MJ, Vasan RS, Carr SA, Wang TJ, Sabatine MS, Clish CB, Gerszten RE. (2010). Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2:33ra37.
- Minami Y, Kasukawa T, Kakazu Y, Iigo M, Sugimoto M, Ikeda S, Yasui A, van der Horst GT, Soga T, Ueda HR. (2009). Measurement of internal body time by blood metabolomics. Proc. Natl. Acad. Sci. U. S. A. 106:9890–9895.
- Nelson W, Tong YL, Lee JK, Halberg F. (1979). Methods for cosinor-rhythmometry. Chronobiologia 6:305–323.
- Nicholson JK, Connelly J, Lindon JC, Holmes E. (2002). Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 1:153–161.
- Otway DT, Mantele S, Bretschneider S, Wright J, Trayhurn P, Skene DJ, Robertson MD, Johnston JD. (2011). Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 60:1577–1581.
- Pandher R, Ducruix C, Eccles SA, Raynaud FI. (2009). Cross-platform Q-TOF validation of global exo-metabolomic analysis: application to human glioblastoma cells treated with the standard PI 3-Kinase inhibitor LY294002. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877:1352–1358.
- Pandher R, Naegele E, Fischer SM, Raynaud FI. (2012). Improvement in the number of analytic features detected by non-targeted metabolomic analysis: influence of the chromatographic system and ionisation technique. In Metabolomics. Rijeka, Croatia: InTech Europe, 317–328
- Pasquali M, Monsen G, Richardson L, Alston M, Longo N. (2006). Biochemical findings in common inborn errors of metabolism. Am. J. Med. Genet. C Semin. Med. Genet. 142C:64–76.
- Portaluppi F, Smolensky MH, Touitou Y. (2010). Ethics and methods for biological rhythm research on animals and human beings. Chronobiol. Int. 27:1911–1929.
- Psychogios N, Hau DD, Peng J, Guo AC, Mandal R, Bouatra S, Sinelnikov I, Krishnamurthy R, Eisner R, Gautam B, Young N, Xia J, Knox C, Dong E, Huang P, Hollander Z, Pedersen TL, Smith SR, Bamforth F, Greiner R, McManus B, Newman JW, Goodfriend T, Wishart DS. (2011). The human serum metabolome. PLoS ONE 6:e16957.
- Santra S, Hendriksz C. (2010). How to use acylcarnitine profiles to help diagnose inborn errors of metabolism. Arch. Dis. Child Educ. Pract. Ed. 95:151–156.
- Sarker D, Workman P. (2007). Pharmacodynamic biomarkers for molecular cancer therapeutics. Adv. Cancer Res. 96:213–268.
- Schofield WN. (1985). Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 39( Suppl 1): 5–41.
- Selmaoui B, Touitou Y. (2003). Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: a study of three different 24-h cycles over six weeks. Life Sci. 73:3339–3349.
- Slupsky CM, Rankin KN, Wagner J, Fu H, Chang D, Weljie AM, Saude EJ, Lix B, Adamko DJ, Shah S, Greiner R, Sykes BD, Marrie TJ. (2007). Investigations of the effects of gender, diurnal variation, and age in human urinary metabolomic profiles. Anal. Chem. 79:6995–7004.
- Walsh MC, Brennan L, Malthouse JP, Roche HM, Gibney MJ. (2006). Effect of acute dietary standardization on the urinary, plasma, and salivary metabolomic profiles of healthy humans. Am. J. Clin. Nutr. 84:531–539.
- Westerhuis JA, van Velzen EJ, Hoefsloot HC, Smilde AK. (2010). Multivariate paired data analysis: multilevel PLSDA versus OPLSDA. Metabolomics 6:119–128.
- Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, Gottfries J, Moritz T, Trygg J. (2008). Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 80:115–122.
- Wurtman RJ, Rose CM, Chou C, Larin FF. (1968). Daily rhythms in the concentrations of various amino acids in human plasma. N. Engl. J. Med. 279:171–175.
- Yamamoto K, Isogai Y, Sato H, Taketomi Y, Murakami M. (2011). Secreted phospholipase A2, lipoprotein hydrolysis, and atherosclerosis: integration with lipidomics. Anal. Bioanal. Chem. 400:1829–1842.
SUPPLEMENTARY FIGURE 1. Venn diagram showing overlapping sets of metabolite features detected by OPLS-DA of paired time points and repeated-measures ANOVA across all time points.
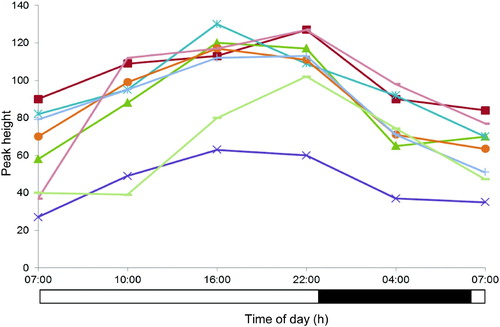
SUPPLEMENTARY FIGURE 2. Plasma profiles of metabolite feature with mass/charge 455.19 Da and retention time 5.7 min for eight individuals detected by repeated-measures ANOVA but not pairwise OPLS-DA comparisons, illustrating higher intersubject variability relative to intrasubject time-of-day variation. All participants were maintained under controlled light/dark, sleep/wake, posture, and calorific intake conditions. Black bar indicates lights-off (0 lux) and white bar lights-on (440–825 lux).
SUPPLEMENTARY TABLE 1. List of metabolite features detected in the screening process and considered for tandem MS
SUPPLEMENTARY TABLE 2. LC-MS features of metabolites of interest that did not exhibit significant 24-h variation (p > .05)