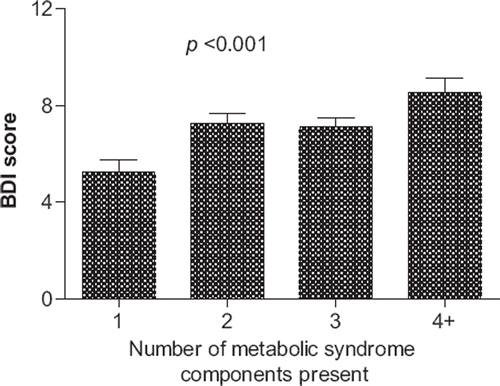Abstract
Background. Both depression and the metabolic syndrome are frequently found among patients with type 1 diabetes, but their potential association has not yet been investigated. In this paper the relationship between depression and the metabolic syndrome among patients with type 1 diabetes was evaluated.
Methods. A total of 1226 patients participating in the Finnish Diabetic Nephropathy Study between 2003 and 2009 were included. Depression was defined as use of antidepressive medication or Beck Depression Inventory (BDI) score ≥16. The metabolic syndrome was defined using the criteria established by the International Diabetes Federation Task Force on Epidemiology and Prevention (IDF); National Heart, Lung, and Blood Institute (NHLBI); American Heart Association (AHA); World Heart Federation (WHF); International Atherosclerosis Society (IAS); and International Association for the Study of Obesity (IASO).
Results. The metabolic syndrome was more frequently observed among depressed patients (57% versus 46%, P = 0.008). Of the individual components of the metabolic syndrome, waist, triglyceride, and HDL components were more frequently fulfilled among patients with depression. The BDI score increased with the number of components of the metabolic syndrome present. The BDI score was independently associated with the waist component (odds ratio 1.03, 95% confidence interval 1.01–1.05) when adjusted for gender, age, socio-economic status, smoking, nephropathy, and HbA1c.
Conclusion. The metabolic syndrome is frequently found among depressed patients with type 1 diabetes. Whether this association influences the development of diabetic complications is not known.
Key words::
| Abbreviations | ||
| AHA | = | American Heart Association |
| BDI | = | Beck Depression Inventory |
| ESRD | = | end-stage renal disease |
| IAS | = | International Atherosclerosis Society |
| IASO | = | International Association for the Study of Obesity |
| IDF | = | International Diabetes Federation Task Force on Epidemiology and Prevention |
| NHLBI | = | National Heart, Lung, and Blood Institute |
| SES | = | Socio-economic status |
| WHF | = | World Heart Federation |
Key messages
The metabolic syndrome is frequently found among depressed patients with type 1 diabetes.
Whether the association between depression and metabolic syndrome influences the development of diabetic complications needs to be elucidated.
Introduction
Diabetes doubles the likelihood of co-morbid depression (Citation1). With observed rates as high as 30%, depression is a major health concern among patients with diabetes. Indeed, the association between depression and diabetic complications is fairly well established (Citation2).
There is increasing evidence that depression is additionally associated with the metabolic syndrome (Citation3,Citation4). The metabolic syndrome represents a cluster of cardiovascular risk factors including increased abdominal obesity, blood pressure, fasting glucose, triglycerides and low high-density lipoprotein (HDL) cholesterol concentration (Citation5). The metabolic syndrome is highly prevalent in Westernized countries, and it can be expected to further increase with the increasing prevalence of obesity (Citation6,Citation7). Moreover, we have previously shown that the metabolic syndrome is also a common finding among patients with type 1 diabetes. Indeed, we observed that 38% of men and 40% of women with type 1 diabetes fulfill three or more components of the metabolic syndrome according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III criteria (Citation8).
Cardiovascular diseases are a major contributor to increased mortality among patients with type 1 diabetes. While nephropathy explains part of this mortality, other candidates likely coexist. Indeed, the metabolic syndrome is associated with increased risk of all-cause and cardiovascular mortality (Citation9–11). As cardiovascular mortality is also associated with depression, it has been speculated that the metabolic syndrome could serve as a link between these two adverse outcomes.
Although there are a number of studies to indicate an association between depression and the metabolic syndrome, the results are not conclusive. In two large studies, for example, no association between depression and metabolic syndrome was observed (Citation12,Citation13). Moreover, some studies have suggested that the association is rather gender-specific (Citation14). Currently, no studies exist regarding a potential association between depression and the metabolic syndrome among patients with type 1 diabetes. Therefore, the aim of the current study was to evaluate whether there is a relationship between the metabolic syndrome and depression among patients with type 1 diabetes.
Patients and methods
All patients were participants of the Finnish Diabetic Nephropathy Study (FinnDiane), a nation-wide, prospective, multicenter study with the aim to identify risk factors for the development of diabetic nephropathy in patients with type 1 diabetes. While data for the FinnDiane Study have been collected since 1998, data collection for psychological determinants did not start until 2003. For the current study, data from all patients with data on depression and components of the metabolic syndrome are presented. In all, cross-sectional data from a total of 1226 patients were available (45% men, mean age 45 ± 12, diabetes duration 28 ± 13). Type 1 diabetes was defined as onset of diabetes before the age of 35 years, permanent insulin treatment initiated within 1 year of diagnosis, and C-peptide negativity. The study protocol was approved by the Ethics Committee of Helsinki University Central Hospital, and a written informed consent was obtained prior to participation.
Symptoms of depression were evaluated with the Beck Depression Inventory (BDI) (Citation14). This self-report questionnaire has 21 items with multiple-choice questions, and the possible scores range from 0 to 63. A cut-off value of ≥16 was used to define depression (Citation15). The BDI scores were additionally treated as continuous variables as appropriate. Moreover, patients using antidepressive medication were also considered depressed. Data on the antidepressive medication utilization was obtained from the Drug Prescription Register of the Social Insurance Institute of Finland.
The metabolic syndrome was defined using the criteria jointly established by International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity (Citation5). According to these criteria, the metabolic syndrome is present when at least three of the following criteria coexist: waist circumference ≥94 cm in men and ≥80 cm in women, triglycerides ≥1.70 mmol/L, HDL cholesterol <1.00 mmol/L in men and <1.30 mmol/L in women, blood pressure ≥130/85 mmHg, and fasting glucose ≥6.11 mmol/L. All patients in the current study were defined to fulfill the criterion for hyperglycemia, while lipid-lowering treatment was not included in the criteria.
Based on medical files, the patients’ attending physician provided information on diabetic complications. Data on smoking and social class (grouped as unskilled/skilled blue-collar, upper/lower white-collar, farmers, and others) were self-reported. Unskilled blue-collar workers were classified as having a low socio-economic status (SES). Blood pressure was measured after a 10-minute rest in a sitting position. Measurement was repeated in 2 minutes, and the mean of the two blood pressure measurements was calculated. Height and weight were measured in light clothing, and body mass index (BMI) was calculated (kg/m2). Waist circumference was measured at the mid-point between the lowest rib and the iliac crest. Serum lipid and lipoprotein concentrations were measured as previously described (Citation8). HbA1c was determined by standardized assays locally.
Renal status was assessed by the urinary albumin excretion rate (AER) in at least two out of three timed 24-h or overnight urine collections. Patients were classified as having normal albumin excretion rate (AER <20 μg/min or <30 mg/24 h), microalbuminuria (AER ≥20 and <200 μg/min, or ≥30 and <300 mg/24 h), macroalbuminuria (AER ≥200 μg/min or ≥300 mg/24 h), or end-stage renal disease (ESRD) (undergoing dialysis or having had a kidney transplant). Diabetic nephropathy was defined as macro albuminuria or ESRD. Data on retinopathy were obtained from medical records. Retinal laser treatment was used as an indication of proliferative retinopathy.
Statistical analyses
Descriptive statistics are reported as percentages for categorical data, mean ± standard deviation for normally distributed data, and median (interquartile range) for non-normally distributed data. The chi-square test, independent sample t test, and Mann-Whitney U test were used for the comparisons of the groups with and without signs of depressive symptoms, as appropriate. Logistic regression analyses were used to explore the variables associated with the BDI score and the metabolic syndrome and its components. The BDI score was entered to the models per unit on the scale. To examine the relationship between the BDI score and the number of components of the metabolic syndrome present, linear regression analysis was performed. A P value <0.05 was considered statistically significant. All data were analyzed using SPSS 15.0 for Windows (SPSS, Chicago, IL).
Results
A total of 214 (17%) patients (14% men and 20% women, P = 0.008) were depressed ( and ). In all, 108 patients used antidepressive medication, 40 of whom scored ≥16 in the Beck Depression Inventory. There was no difference in the frequency of observed metabolic syndrome between depressed patients with (59%) and without (55%) antidepressive medication. Moreover, those with antidepressive medication, as opposed to those without, did not fulfill the criteria for waist (66% versus 60%), HDL (22% versus 18%), or blood pressure (69% versus 67%) more frequently. However, the triglyceride criterion was less frequently fulfilled among those with antidepressive medication (14% versus 31%, P = 0.007). Of the antidepressive medication, selective serotonin reuptake inhibitors (SSRI) were most frequently used (60%), followed by tricyclic medication (21%) and other type of medication (19%). Patients with different types of antidepressive medication did not differ with respect to the frequency of fulfilling the criteria for the metabolic syndrome or any of its components.
Table I. Patient characteristics, men and women combined.
Table II. Patient characteristics.
Depressed patients were older and had longer diabetes duration than those not depressed (). More over the frequencies of nephropathy and proliferative retinopathy were higher among depressed patients. Depressed patients were also more frequently smokers, had low SES and were less frequently employed. The metabolic syndrome was more frequently observed among depressed patients (57% versus 46%, P = 0.008). Of the individual components of the metabolic syndrome, the waist, triglyceride, and HDL components were more frequently fulfilled among those with depression. Moreover, depressed patients had higher serum triglyceride concentrations.
In women, the metabolic syndrome was more frequently observed among those depressed (54% versus 43%) (). Although not significant, depressed men also showed somewhat higher frequency of metabolic syndrome (63% versus 50%). Furthermore, separate analyses among men and women revealed that the more frequently fulfilled waist and HDL components in depressed patients were due to the differences in women, while the triglyceride component was more prevalent in depressed men.
In logistic regression analyses, BDI score was independently associated with waist component (odds ratio 1.03, 95% confidence interval 1.01–1.05), and triglyceride component (OR 1.02, 95% CI 1.01–1.05) when adjusted for gender, age and socio-economic status (). The BDI score remained associated with waist component after further adjustment with smoking, nephropathy, and HbA1c. The results from unadjusted models (data not shown) did not differ from those observed in the model 1. Model 3 was also analyzed entering the depression to the model as a dichotomous variable. In these analyses, depression was not associated with any of the variables of the metabolic syndrome.
Table III. Beck Depression Inventory score and metabolic syndrome.
Compared to patients with the metabolic syndrome, the ones without had lower BDI scores (4 (Citation1–9) versus 5 (Citation2–11), P = 0.001). The BDI score increased with the number of the metabolic syndrome components present (P < 0.001) ().
Discussion
This study provides new evidence that there is an association between depression and the metabolic syndrome among patients with type 1 diabetes. The metabolic syndrome was observed more frequently among the depressed patients. Of the individual components of the metabolic syndrome, the waist, triglyceride, and HDL components were more frequently fulfilled among depressed individuals. Higher BDI scores were observed among patients with the metabolic syndrome, and furthermore the BDI scores were found to increase with the number of the components of metabolic syndrome present.
While, to our knowledge, this is the first study to assess a potential association between depression and the metabolic syndrome in patients with type 1 diabetes, our results are in concordance with many studies conducted with respect to other entities. Skilton et al., for example, observed an association between the metabolic syndrome and depressive symptoms among subjects with an increased risk of cardiovascular disease (Citation16). Moreover, using the Hospital Anxiety and Depression scale, they found the depression scores to increase as the number of components of the metabolic syndrome increased. In two large studies in the general population, however, no association between depression and metabolic syndrome was observed (Citation12,Citation13). While in the first of these studies the lack of association could have been due to a selective inclusion of young adults and subsequently low rate of observed metabolic syndrome (Citation12), the results of the second study by Hildrum et al. seem more generalizable (Citation13).
In the current study, the metabolic syndrome was more frequently observed among depressed women. Such a difference was not observed in men. A number of studies have suggested that the association between depression and the metabolic syndrome or its components is mainly present in women. In a fairly large study in apparently healthy employed participants, depression among women, but not men, was associated with an almost 2-fold increased risk of having the metabolic syndrome, and more than a 2-fold increased risk of fulfilling the waist and glucose components (Citation14). Although depression also increased the risk of fulfilling the waist component among men, Toker et al. speculated that their findings may indicate that the association between the metabolic syndrome and depression is gender-specific (Citation14). This observation was further supported by Muhtz et al. who, in their study among 215 healthy adults from the general population, did not find any of the components of the metabolic syndrome to be associated with depression among men, while depressed women had larger waist circumference, higher fasting blood glucose concentration, lower HDL concentration, and higher diastolic blood pressure (Citation17). In the current study, analyzing men and women separately, the waist and HDL components were observed to be associated with depression only among women, while the triglyceride component was associated with depression only in men.
One of the possible reasons for the observed gender differences in depression and metabolic syndrome may be that women, compared to men, tend to express more depressive symptoms (Citation18). Indeed, this was also found in the current study, as depression was observed in 14% and 20% of men and women, respectively.
There are both physiological and behavioral pathways that could explain the association between depression and metabolic syndrome. First, as found in depression, chronic dysregulation of the hypothalamic pituitary adrenal axis can result in excess glucocorticoid secretion, which, if sustained over time, contributes to hypertension, insulin resistance, visceral obesity, and increased lipid concentrations (Citation19). Second, reduced brain serotonin activity contributes both to the metabolic syndrome (Citation20) and depression (Citation21) and could therefore serve as a mediating link between the two conditions. Third, depression is associated with poor compliance with self-management practices (Citation22), giving rise to detrimental consequences such as increased central adiposity. Indeed, obesity has been associated with depression in a number of studies (Citation23–25). Our results are in agreement with these studies as the BDI score was associated with the waist component of the metabolic syndrome.
One of the underlying mechanisms in the association between increased adiposity and depression could be the use of antidepressive medication. Williams et al. reported, however, that the associations between depression and adiposity were not attenuated by adjustment for psychotropic medication use (Citation24). Similarly, in the current study, the use of antidepressive medication among those with depression was not associated with the frequency of observed metabolic syndrome. Moreover, there were no differences in the frequencies of metabolic syndrome or its components between patients using either tricyclic, SSRI, or other antidepressive medication.
In the current study, depression was defined as the use of antidepressive medication or having the BDI score ≥ 16. However, antidepressive medication may also be prescribed for a number of other indications. Data regarding indications were not available in the current study and therefore some misclassification of patients could have taken place. Moreover, the use of the BDI instead of a diagnostic interview may partially misclassify the patients, and the results have to be interpreted in the light of this limitation. The BDI has, however, been validated as a tool to measure depression in patients with diabetes, and the currently used cut-off point has been shown to provide a good balance between sensitivity and specificity (Citation26) and to reduce the potential for misclassification.
Depression is frequently found among patients with diabetes. Indeed the odds of depression among patients with diabetes are twice that of the healthy individuals (Citation1). Moreover, depression is also associated with increased risk of diabetic complications (Citation2). While the metabolic syndrome is frequently observed also among patients with type 1 diabetes, its significance for these patients has only recently attracted interest. In our previous study among patients with type 1 diabetes, we found that the metabolic syndrome is an additional risk factor, beyond albuminuria, as it caused a 2.1-fold increase of cardiovascular events and a 2.5-fold increased risk of cardiovascular and diabetes-related mortality (Citation27). Similarly, McGill et al. observed that patients with type 1 diabetes and the metabolic syndrome are more prone to develop micro- and macrovascular complications (Citation28). Whether depression increases the risk of long-term complications via components of the metabolic syndrome has to be further studied. Moreover, the possibility that treatment of the depression could reduce the presence of components of the metabolic syndrome should also be investigated.
A major limitation is the cross-sectional nature of this study that prevents us from drawing conclusions about any causal relationships between depression and the metabolic syndrome. Causality, as well as the consequences of depression and the metabolic syndrome with respect to mortality and morbidity, has to be further elucidated.
In conclusion, the metabolic syndrome is frequently found among depressed patients with type 1 diabetes. The association between depression and the metabolic syndrome, and their potential influence on the development of diabetic complications, has to be studied in the prospective setting.
Supplementary appendix
Download PDF (38.8 KB)Acknowledgements
This study was supported by grants from the Signe and Ane Gyllenberg Foundation, Folkhälsan Research Foundation, and Wilhelm and Else Stockmann Foundation. The skilled technical assistance of Anna Sandelin, Jaana Tuomikangas, Valma Harjutsalo, and Jessica Thorn is gratefully acknowledged. Finally, the authors acknowledge all the physicians and nurses at each center participating in the collection of patients (online appendix).
Declaration of interest: The authors declare that there is no duality of interest associated with this manuscript.
References
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–78.
- de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63: 619–30.
- Miettola J, Niskanen LK, Viinamäki H, Kumpusalo E. Metabolic syndrome is associated with self-perceived depression. Scand J Prim Health Care. 2008;26:203–10.
- Räikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism. 2002;51:1573–7.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5.
- Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005;28:2745–9.
- Hu G, Lindström J, Jousilahti P, Peltonen M, Sjöberg L, Kaaja R, . The increasing prevalence of metabolic syndrome among Finnish men and women over a decade. J Clin Endocrinol Metab. 2008;93:832–6.
- Thorn LM, Forsblom C, Fagerudd J, Thomas MC, Pettersson-Fernholm K, Saraheimo M, . Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care. 2005; 28:2019–24.
- Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008; 31:1898–904.
- Ho JS, Cannaday JJ, Barlow CE, Mitchell TL, Cooper KH, FitzGerald SJ. Relation of the number of metabolic syndrome risk factors with all-cause and cardiovascular mortality. Am J Cardiol. 2008;102:689–92.
- Hildrum B, Mykletun A, Dahl AA, Midthjell K. Metabolic syndrome and risk of mortality in middle-aged versus elderly individuals: the Nord-Trondelag Health Study (HUNT). Diabetologia. 2009;52:583–90.
- Herva A, Räsänen P, Miettunen J, Timonen M, Läksy K, Veijola J, . Co-occurrence of metabolic syndrome with depression and anxiety in young adults: the Northern Finland 1966 Birth Cohort Study. Psychosom Med. 2006;68: 213–6.
- Hildrum B, Mykletun A, Midthjell K, Ismail J, Dahl AA. No association of depression and anxiety with the metabolic syndrome: the Norwegian HUNT study. Acta Psyhciatr Scand. 2009;120:14–22.
- Toker S, Shirom A, Melamed S. Depression and the metabolic syndrome: gender-dependent associations. Depress Anxiety. 2008;25:661–9.
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71.
- Skilton MR, Moulin P, Terra JL, Bonnet F. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry. 2007;62:1251–7.
- Muhtz C, Zyriax BC, Klahn T, Windler E, Otte C. Depressive symptoms and metabolic risk: effects of cortisol and gender. Psychoneuroendocrinology. 2009;34:1004–11.
- Grigoriadis S, Robinson GE. Gender issues in depression. Ann Clin Psychiatry. 2007;19:247–55.
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annu Rev Public Health. 2005;26: 469–500.
- Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. J Clin Endocrinol Metabol 2006;91: 718–21.
- Heltzer H. Serotonergic dysfunction in depression. Br J Psychiatry. 1989;155:25–31.
- Katon WJ. The comorbidity of diabetes mellitus and depression. Am J Med. 2008;121:S8–15.
- Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64:97–105.
- Williams LJ, Pasco JA, Henry MJ, Jacka FN, Dodd S, Nicholson GC, . Lifetime psychiatric disorders and body composition: a population-based study. J Affect Disord. 2009;118:173–9.
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, . Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30.
- Lustman PJ, Clouse RE, Griffith LS, Carney RM, Freedland KE. Screening for depression in diabetes using the Beck Depression Inventory. Psychosom Med. 1997;59:24–31.
- Thorn LM, Forsblom C, Wadén J, Saraheimo M, Tolonen N, Hietala K, . Metabolic syndrome as a risk factor for cardiovascular disease, mortality, and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care. 2009;32:950–2.
- McGill M, Molyneaux L, Twigg SM, Yue DK. The metabolic syndrome in type 1 diabetes: does it exist and does it matter? J Diabetes Complications. 2008;22:18–23.