Abstract
Background. Serum lipoproteins, the carriers of cholesterol and other lipophilic substances in blood, are known to contain variable amounts of lipid peroxides. We investigated the transport of food-derived and endogenously formed lipid peroxides by serum lipoproteins under physiological conditions.
Methods. Five independent trials were conducted in which different groups of healthy volunteers either consumed a test meal (a standard hamburger meal rich in lipid peroxides) or underwent strenuous physical exercise. The transport function was characterized by analyzing the kinetics of lipid peroxides in lipoprotein fractions. For evaluation of their potential involvement, indicators of oxidative stress (8-isoprostanes, malondialdehyde, 8-oxo-deoxyguanosine), antioxidant functions (total antioxidant potential, paraoxonase activity), and serum lipids were also analyzed.
Results. We found that food lipid peroxides are incorporated into serum triglyceride-rich lipoproteins and low-density lipoprotein, directing the flow of lipid peroxides towards peripheral tissues. High-density lipoprotein appears to have an opposite and protective function, and is able to respond to oxidative stress by substantially increasing the reverse transport of lipid peroxides.
Conclusions. We propose that the specific atherosclerosis-related effects of serum lipoproteins are not explained by cholesterol transport alone and may rather result from the transport of the more directly atherogenic lipid peroxides.
| Abbreviations | ||
| ABAP | = | 2,2′azobis(2-amidinopropane)HCl |
| DG | = | deoxyguanosine |
| HDL | = | high-density lipoproteins |
| LDL | = | low-density lipoproteins |
| 8-oxo-dG | = | 8-oxo-deoxyguanosine |
| TRL | = | triglyceride-rich lipoproteins |
Key messages
Serum lipoproteins are active transporters of circulating lipid peroxides.
The specific roles of serum lipoproteins in atherosclerosis may in part be explained by the transport of atherogenic lipid peroxides.
Introduction
Lipoprotein particles transport lipids and lipid-soluble material in the blood-stream to and from the liver. Low-density lipoprotein (LDL) is the main transporter of cholesterol to the peripheral tissues, while excess tissue cholesterol is returned to the liver by reverse cholesterol transport mediated by high-density lipoprotein (HDL). In parallel with the transport functions, high LDL cholesterol is associated with elevated risk of cardiovascular disease, while high HDL cholesterol appears to be protective (Citation1). In addition to cholesterol and other lipophilic substances, serum lipoproteins are known to contain variable amounts of lipid peroxides.
In-vitro studies have indicated that dietary oxidized lipids can be absorbed and further esterified to complex lipids by intestinal epithelial cells (Citation2). In accordance, Strapans and co-workers (Citation3) found that oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. We have previously shown that lipid peroxides present in food are incorporated into chylomicron, VLDL, and LDL particles of pigs in a dose-dependent manner (Citation4). Analysis with reverse phase HPLC and electrospray ionization-MS demonstrated that the oxidatively modified lipoprotein lipids typically consisted of hydroxy-, epoxy-, and keto-derivatives of fatty acids, and of triacylglycerol core aldehydes. Together these findings seem to indicate that food lipid peroxides become integral parts of lipoprotein particles and suggest that serum lipoproteins act as carriers not only for native (non-oxidized) lipids but also for a variety of lipid peroxidation products.
Oxidized lipids can have wide-spread effects on normal physiological functions. In particular, the association between LDL-oxidized lipids and atherosclerosis seems strong: LDL-oxidized lipids are directly linked with macrophage accumulation, regulation of macrophage activity, and foam cell formation in vessel wall (Citation5), and oxidized lipids are strongly involved in activation of atherosclerosis-related gene groups (Citation6). Oxidized lipids in circulating LDL are known to be strongly associated with coronary (Citation7), carotid (Citation8,Citation9), and brachial (Citation8) atherosclerosis, hypertension (Citation8), and arterial functions (Citation9,Citation10). In line with the beneficial effects of risk management programs, serum concentrations of LDL-oxidized lipids can be reduced by weight reduction (Citation11,Citation12), physical activity (Citation13,Citation14), statin treatment (Citation15–17), and dietary interventions (Citation15,Citation18). Common to all these studies, LDL-oxidized lipids seem to be more closely related to atherosclerosis than the customary lipid markers.
Thus far, the presence of oxidized lipids in serum lipoproteins has been merely regarded as a general consequence of oxidative stress, and the transport of lipid peroxides by serum lipoproteins has received little attention. In the present study we investigated, under physiological conditions where blood lipid peroxide concentrations are increased, the transport of food-derived and endogenously formed lipid peroxides by serum lipoproteins.
Materials and methods
Chemicals
Chloroform (p.a.), methanol (p.a.), and cyclohexane (Uvasol) were from E. Merck (Darmstadt, Germany), heparin from Lövens Kemiska Fabrik (Ballerup, Denmark). 5-Amino-2,3-dihydro-1,4-phthalazinedione (Luminol) was purchased from Bio-Orbit Ltd (Turku, Finland), 2,2′azobis(2-amidinopropane)HCl (ABAP) from Polysciences Inc. (Warrington, PA, USA), and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) from Aldrich Chemicals Co. (Milwaukee, WI, USA). Alkaline phosphatase was from Finnzymes (Espoo, Finland). All other reagents were from Sigma Chemicals Co. (St. Louis, MO, USA).
Subjects
Five independent trials were conducted in which different groups of healthy volunteers either consumed a test meal or underwent strenuous physical exercise. In trials 1–3, healthy volunteers consumed a test meal, after which the postprandial responses were followed for 6 h by blood tests. In trials 4 and 5 the subjects performed a controlled physical exercise, and blood samples were taken before, during, and after the exercise. The study protocols were approved by the local ethics committees. The subjects in trial 1 (n = 10) were mildly hyperlipidemic (serum total cholesterol 6.2 ± 1.3 mmol/L; triglycerides 2.8 ± 1.0 mmol/L) and overweight (body mass index (BMI) 28 ± 2) men (). The subjects in trial 2 (n = 36) were normolipidemic men and women. Subjects were included to the dietary studies if they were healthy, aged between 20 and 50 years, BMI was under 30, if they were omnivorous, and were not participating any other study. Exclusion criteria were obesity (BMI > 30), any chronic diseases, all medications, and acute gastrointestinal disorders 2 months prior to the study which had required medication. In trial 3, 11 men were recruited using the same criteria as above (). The subjects in exercise trials 4 and 5 were healthy male volunteers. Subjects of trial 4 (n = 20) consisted of ten top-level middle-distance and ten marathon runners (). The subjects in trial 5 (n = 12) were keep-fit runners ().
Table I. Base-line characteristics of the participants.
Test diets
Before the study the subjects were asked to maintain their normal life-style habits and not to make any changes to the diet or in the physical activity. The test subjects had a standardized breakfast in the morning at 8 o'clock. The subjects were not allowed to eat anything until 11 o'clock when the base-line (0 min) blood sample was drawn. After the base-line blood sample, subjects in trials 1 and 2 consumed a standard hamburger meal () and 4 dL of fruit juice. Further blood samples were taken at 30, 60, 120, 240, and 360 min time points after base-line measurement. The subjects were not allowed to eat, but they were allowed to drink water during the 6-h test period. The test meal in trial 3 was ordinary oatmeal porridge (). In this study blood samples were taken before and 120 and 240 min after the meal.
Table II. Basic nutritional characteristics of test meals.
Acute physical exercise protocols
In trial 4, the subjects performed on a treadmill a 40 min tempo run at the velocity corresponding to 80% VO2max after a 40-min warm-up (Citation19). Venous blood samples were taken before exercise, after 20 min of exercise, and immediately, 15 min, and 90 min after the exercise. In trial 5, the exercise test consisted of a 20-km continuous run (at a velocity corresponding to 70% VO2max) followed immediately by a test run, where the velocity was conducted to increase 0.1 m/s until exhaustion. Blood samples were taken before the exercise, after 20 km continuous running, and immediately and 90 min after the exhaustive test run. In both exercise protocols the subjects were allowed to drink only water during the exercise test days. On the test day the subjects consumed a standardized, light breakfast 3 h before the exercise.
Determination of oxidized lipoprotein lipids
Analysis of lipoprotein-oxidized lipids was based on determination of the base-line level of conjugated dienes in lipoprotein lipids (Citation20). Appearance of conjugated dienes has been commonly used as the index of oxidation in in-vitro and ex-vivo studies on LDL oxidation. Serum triglyceride-rich lipoproteins (TRL) were isolated from serum samples by spinning at 15,500 g for 20 min at 12°C (Citation21). Serum LDL was isolated by precipitation with buffered heparin (Citation20). Isolation of the HDL fraction from serum samples was based on phosphotungstic acid precipitation (Citation22). The isolation procedures were validated for the purpose and did not affect the level of oxidized lipids (Citation20). Lipids were extracted from isolated lipoproteins by chloroform-methanol (2:1), dried under nitrogen, and redissolved in cyclohexane. The amount of peroxidized lipids in lipoprotein lipids was assessed spectrophotometrically as the amount of diene conjugation (234 nm). Validation studies for the assay have ruled out interference by non-specific substances and shown that diene conjugation is a measure of oxidative LDL modification found in all LDL lipid classes. In addition to the specific absorption spectra at 234 nm, the presence of conjugated dienes has been verified by NMR studies (Citation7). The coefficient of variation (CV) for within-assay precision for determination of oxidized lipoprotein lipids was 4.4%, and the CV for the between-assay precision was 4.5%. Malondialdehyde concentration was measured as serum total (free and protein-bound) malondialdehyde as the 2,4-dinitrophenylhydrazine derivative by HPLC with 1,1,3,3-tetraethoxypropane as the standard (Citation23). The postprandial lipoprotein lipids were also analyzed after separation of lipid classes by HPLC. The HPLC analyses were performed with a Shimadzu 10ADVP. Luna 5 μ silica column, 250 × 4.6 mm, was used, and the detection of oxidized lipoprotein lipids was based on UV detector operating at 234 nm. The eluent was 4% propanol in hexane, and the flow rate was 1 mL/min. Lipid classes were located by the HPLC elution volume correlations with standard samples.
Other analytical procedures
Serum total (free and esterified) 8-isoprostanes were used as an indication of overall oxidative stress and determined by a competitive enzyme immunoassay Isoprostane EIA kit (Cayman Chemical Company, Ann Arbor, MI, USA). 8-Oxo-deoxyguanosine (8-oxo-dG) was determined as an indication of oxidative DNA modification. DNA from whole blood was isolated by a non-enzymatic method (Citation24). The amount of 8-oxo-dG was determined using HPLC equipped with an electrochemical detector, and deoxyguanosine (dG) was determined with UV detector. The 8-oxo-dG concentration was expressed as the ratio of 8-oxo-dG per 105 dG. Total peroxyl radical-trapping antioxidant potential was estimated ex vivo by the potency of serum samples to resist ABAP-induced peroxidation. Trolox served as a standard radical scavenger. Paraoxonase activity was determined using paraoxon (O,O-diethyl-O-p-nitrophenylphosphate) as the substrate (Citation25). Serum triacylglycerols, total cholesterol, LDL cholesterol, HDL cholesterol, and glucose were measured using standard enzymatic methods (Roche Diagnostics, Mannhein, Germany). HDL cholesterol concentration was measured after phosphotungstic acid precipitation. Serum insulin was measured with time-resolved fluoroimmunoassay method using Wallac AutoDELFIA analyzer (Wallac, Turku, Finland).
Analysis of lipid peroxide contents of meals
The meals were weighed whereafter they were minced and homogenized. Lipids were extracted with chloroform-methanol (2:1). The extract was evaporated to dryness and redissolved in cyclohexane. Measurement of the lipid peroxide content was based on spectrophotometric determination of diene conjugation at 234 nm. Absorbance units were converted to molar units using the molar extinction coefficient 2.95 × 104 M−1 cm−1.
Statistical analysis
We used Statistical Package for Social Sciences (SPSS) version 14.0. The differences were tested using the general linear model procedure for repeated measures. In case of significant time effect, a dependent samples t test was used to reveal differences between the base-line and postprandial/exercise samples. Pearson correlation analyses were used to reveal associations between parameters. We used logarithmically transformed values of the variables that had a significantly skewed distribution. A P value < 0.05 was considered statistically significant.
Results
Transport of food-derived lipid peroxides
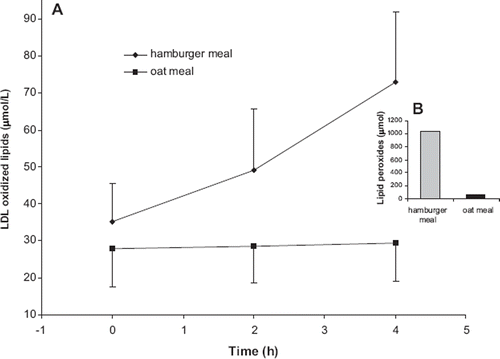
A standard hamburger meal served as the source of food-derived lipid peroxides in the present study. The high lipid peroxide content of the meal was verified by prior analysis (). We found (trial 1) that lipophilic lipid peroxides in TRL and LDL were elevated within 1 h after the meal and remained elevated throughout the postprandial period. The increase was more pronounced in TRL and was maximal for both TRL (2.7-fold) and LDL (2.1-fold) 4 h after the meal (). When the lipoprotein lipids were analyzed after HPLC separation of lipid classes, it was found that practically all of the postprandially increased lipid peroxides in TRL (96%) and LDL (91%) were in the triglyceride and cholesteryl ester fractions (data not shown). When compared to TRL and LDL, a much slower and less prominent meal-induced increase (1.2-fold) was seen in HDL lipophilic lipid peroxides, where the levels were significantly elevated not until 6 h after the meal ().
Figure 1. Lipophilic lipid peroxides (A) and malondialdehyde (B) concentrations in serum lipoproteins after consumption of a meal rich in lipid peroxides. Lipid peroxidation products were analyzed in blood samples of healthy volunteers (trial 1, n = 10) during the post prandial period after consumption of a standard hamburger meal. LDL = low-density lipoproteins; TRL = triglyceride-rich lipo proteins; HDL = high-density lipoproteins. Mean ± SD are indicated. *Statistically different from 0 h time point, P < 0.05; **P < 0.01; ***P < 0.001.
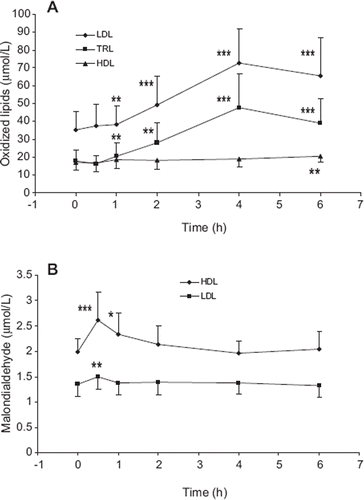
In comparison to the lipophilic lipid peroxides, the lipoprotein accumulation and disappearance of the water-soluble lipid peroxidation product malondialdehyde (MDA) was quite different: MDA was preferentially sequestered by HDL, and the lipoprotein accumulation of MDA was very rapid, being maximal 30 min after the meal ().
We then repeated the study with an increased number of participating subjects, and evaluated the lipid peroxide carrier function of lipoproteins in relation to the postprandial transport of native lipids, oxidative stress, and antioxidant functions (trial 2). We found that in addition to the concentration of LDL-oxidized lipids, the ratio of LDL-oxidized lipids:LDL cholesterol was markedly elevated throughout the postprandial period (). Serum total, LDL and HDL cholesterol concentrations were not changed. As expected, serum triglyceride and insulin concentrations increased in response to the hamburger meal ().
Table III. Effect of hamburger meal on serum lipids and indicators of oxidative stress in trial 2.a
There were only modest changes in markers of the oxidative stress (serum 8-isoprostane and malondialdehyde concentrations, 8-oxo-deoxyguanosine in white blood cell DNA; ). Moreover, serum total antioxidant potential and paraoxonase activity were not found to be affected by the test meal ().
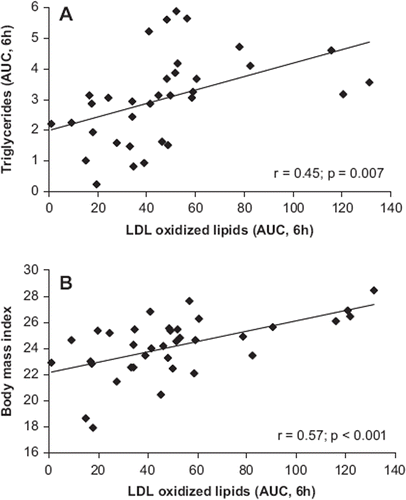
Correlation analysis showed that the incremental area under the curve (AUC) values for LDL-oxidized lipids were associated with the corresponding serum triglyceride values () and BMI () of the participants.
Figure 2. Correlation of the AUC for LDL-oxidized lipids with that of serum triglyceride concentration (A) and with the body mass index (BMI) (B). The data are based on analyses made in blood samples of healthy volunteers (trial 2, n = 36) during the postprandial period after consumption of a standard hamburger meal.
The postprandial effects of the lipid peroxide-rich hamburger meal were compared to those of an oat meal, which was shown to contain only a small amount of lipid peroxides (, ). We found that consumption of the oat meal had no effect on postprandial levels of oxidized LDL lipids ().
Clearance of lipid peroxides by HDL
Subjects in trial 2 with low or high basal HDL cholesterol were compared to each other with respect to the meal-induced response in LDL-oxidized lipids and triglycerides (expressed as the incremental area under the curve, AUC). It was found that among subjects with low basal HDL, the AUC for LDL-oxidized lipids was considerably higher compared to those with high basal HDL. Yet, the basal HDL level was not related to the postprandial clearance of triglycerides ().
Table IV. Effect of HDL level on meal-induced postprandial responses in LDL-oxidized lipid and triglyceride concentrations.a
Transport of endogenously formed lipid peroxides
The transport of endogenously formed lipid peroxides was studied in a physiological model of oxidative stress induced by physical exercise. The study group in trial 4 consisted of well trained endurance runners, who performed a 40 min treadmill run. The exercise clearly elevated oxidative stress of subjects, as verified by the increased concentration of serum malondialdehyde (before run MDA: 1.72 ± 0.71; after run 2.41 ± 1.12 μmol/L; P < 0.001). The physical exercise-induced oxidative stress did not affect the amount of LDL-oxidized lipids (). Contrary to this, the lipid peroxide load of HDL was substantially (26%) increased, and was elevated still 90 min after the exercise (). In addition to the total amount of HDL-oxidized lipids, also the ratio of HDL-oxidized lipids:HDL cholesterol was increased. Acute exercise did not affect serum paraoxonase activity (), but paradoxically slightly increased serum total antioxidant potential (data not shown). When the HDL-oxidized lipids were analyzed after separation of lipid classes by HPLC, it was found that the increase was mainly due to oxidized cholesteryl esters and, to a smaller extent, oxidized phospholipids ().
Figure 4. The effect of acute physical exercise (trial 4, n = 20) on the concentration of LDL-oxidized lipids and paraoxonase activity (A), and on the concentration of HDL-oxidized lipids (B). The effect of physical exercise on lipid peroxide concentrations in various HDL lipid fractions (C). The effect of intensity of the physical exercise (trial 5, n = 12) on the level of HDL-oxidized lipids (D). Mean ± SD are indicated. ***Statistically different from the pre-exercise value, P < 0.001. Different letters indicate significant differences between the time points (P < 0.05).
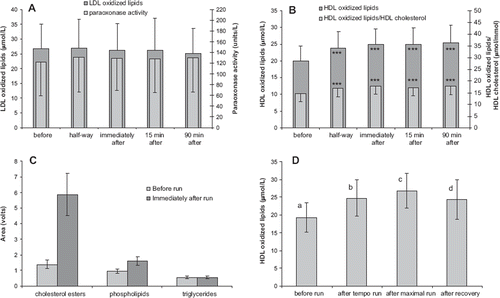
The exercise study was repeated in a slightly differing model of physical activity with another group of endurance runners (trial 5). In addition to confirming the previous result, it was demonstrated that accumulation of lipid peroxides in HDL was dependent on the intensity of the physical activity ().
Discussion
The lipoprotein-specific responses to food-derived and endogenous lipid peroxides show that lipoprotein lipid peroxide levels are not only a random consequence of oxidative stress, but rather indicative of specific lipid peroxide transport functions. Consumption of a meal rich in lipid peroxides elevated substantially the lipid peroxide concentration in LDL. On the contrary, endogenous lipid peroxides generated by physical exercise were not taken up by LDL. This points to a transport mechanism where LDL acts as a vehicle for exogenous lipid peroxides and, in parallel with the cholesterol-transport function, carries lipid peroxides towards peripheral tissues. This would mean a potential pro-oxidant role for LDL in the body. Importantly, our results would also mean that a fatty meal rich in lipid peroxides can directly cause atherogenic LDL modifications. Thus far, it has been generally assumed that oxidatively modified LDL is exclusively due to endogenous free radical reactions induced e.g. by metal ions, superoxide anion, nitric oxide, lipoxygenase, or myeloperoxidase (Citation26). In accordance with our results, recent experimental evidence shows that postprandial LDL is more atherogenic than LDL in fasting conditions (Citation27), an effect postulated to be mediated by lipid peroxides.
The lipid peroxide content of the hamburger meal was 1030 μmol (). In UK the average daily intake of lipid hydroperoxides associated with fats and oils was estimated to be in the region of 1500 μmol per day (Citation28). It is therefore possible that at the average level of fat consumption a substantial part of LDL-oxidized lipids could be of dietary origin. Concluding from previous studies on risk management programs, the magnitude of the hamburger meal-induced increase in LDL-oxidized lipids was significant, comparable to that caused by 10 kg overweight (Citation29).
The postprandial rise in LDL-oxidized lipids appeared to be related to the lipid peroxide content of the meal, as indicated by the comparison of effects of the hamburger meal and oat meal. These data, however, lack credibility due to the limited number and poor comparability of the test meals. Yet, the finding does tell that an elevation in LDL-oxidized lipids is not an inevitable physiological response to any meal, but depends on the nature of the ingested food.
The meal-induced increase in LDL-oxidized lipids could not be explained by an increase in LDL cholesterol concentration, as indicated by the fact that LDL cholesterol was not affected by the meal. The results further showed that the increase in LDL-oxidized lipids was neither due to acute meal-induced oxidative stress, nor to impairment of antioxidant defense functions. In contrast to the rise in LDL-oxidized lipids as reported in the present study, the level of oxidatively modified apolipoprotein B was found in a previous study to be slightly decreased after a high-fat meal (Citation30).
The fatty meal-induced increase in LDL-oxidized lipids was associated with a simultaneous increase in triglyceride level and also with the BMI of the participants. These findings are most interesting in light of the recent evidence which shows that postprandial triglyceride levels have a determining role in cardiovascular disease (31,32), and LDL-oxidized lipids have adipose tissue mass modulating effects (Citation33).
Results of the present study draw attention to another type of dietary risk factors of atherosclerosis, lipid peroxides formed e.g. during food storage, processing, or digestion, emphasizing the importance of pro/antioxidant effects of nutrients. In support of this, epidemiological studies show that low levels of dietary antioxidants are associated with increased risk for cardiovascular disease and that increased intakes appear to be protective (Citation34). Supplemental antioxidant preparations, especially if not taken together with meals, likely have little effect on the presence or formation of lipid peroxides in the alimentary tract. In accordance, antioxidant supplementation did not affect the concentration of LDL-oxidized lipids, even though the LDL antioxidant potential was substantially increased (Citation35).
We found that endogenous oxidative stress, as induced by strenuous physical activity, resulted in a rapid elevation of lipid oxidation products in HDL particles. It is unlikely that the exercise-induced increase of lipid peroxides in HDL particles would have been due to oxidation of the HDL lipids since the LDL lipids were not oxidized under these conditions.
The HDL-specific increase of lipid peroxide concentrations during endogenous oxidative stress and the fact that high HDL level was associated with a more efficient clearance of the meal-induced lipid peroxide load of LDL are indicative of lipid peroxide clearance function of HDL. It has been shown earlier in a perfused rat liver model that the liver removes oxidized cholesteryl esters from HDL but not those from the LDL particles (Citation36). Moreover, Fluiter et al. (Citation37) showed that there is a preferential liver uptake, coupled to a rapid biliary excretion pathway, of oxidized cholesteryl esters in HDL, as compared with the unoxidized cholesteryl esters. This would mean that the protective transport function of HDL is not limited to removal of excess cholesterol but covers also the noxious lipid peroxides. The significance of lipid peroxide transport for antioxidant protection of the body remains to be elucidated.
At present, two commonly accepted theories explain the roles of LDL and HDL in atherosclerosis. In addition to the cholesterol transport function, the noxious effects of LDL are explained by the oxidation theory of atherosclerosis, according to which ‘oxidative modification of LDL is important and possibly obligatory in the pathogenesis of the atherosclerotic lesion’ (Citation38). The beneficial effects of HDL have been explained by the mechanism of reverse cholesterol transport. Our data on lipid peroxide transport are in full agreement with both oxidation and reverse transport theories and, in fact, not only expand but also combine the two theories in a logical way.
Conclusions
We found evidence for another lipoprotein transport function with potentially high physiological significance. We found that serum lipoproteins are active carriers of products of lipid peroxidation, LDL and TRL directing transport towards peripheral tissues, and HDL being active in the reverse transport of lipid peroxides. Indeed, our data combine in a rational way the ‘oxidation’ and ‘reverse cholesterol transport’ theories of atherosclerosis. Thus far the risk-determining role of serum lipoproteins has been explained by cholesterol transport. Since lipid peroxides are well known harmful species which in experimental studies directly stimulate atherosclerotic processes (Citation26), it is possible that the lipid peroxide transport function will eventually turn out to be an important risk-related mechanism in the development of atherosclerosis.
Acknowledgements
We thank Ms Leena Söderholm and Ms Niina Hallikainen for excellent technical assistance.
Declaration of interest: This study was supported by grants from the Finnish Funding Agency for Technology and Innovation, and from the Finnish Cultural Foundation. M Ahotupa is the inventor for patents and patent applications related to assays for lipoprotein lipids. The other authors declare no other conflicts of interest.
References
- Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, . HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357:1301–10.
- Penumetcha M, Khan N, Parthasarathy S. Dietary oxidized fatty acids: an atherogenic risk? J Lipid Res. 2000;41:1473–80.
- Strapans I, Rapp JH, Pan XM, Feingold KR. Oxidized lipids in the diet are a source of oxidized lipid in chylomicrons of human serum. Arterioscler Thromb. 1994;14:1900–5.
- Suomela J-P, Ahotupa M, Kallio H. Triacylglycerol oxidation in pig lipoproteins after a diet rich in oxidized sunflower seed oil. Lipids. 2005;40:437–44.
- Birukov KG. Oxidized lipids: the two faces of vascular inflammation. Curr Atheroscler Rep. 2006;8:223–31.
- Furnkranz A, Schober A, Bochkov VN, Bashtrykov P, Kronke G, Kadl A, . Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler Thromb Vasc Biol. 2005;25:633–8.
- Vasankari T, Ahotupa M, Toikka J, Mikkola J, Irjala K, Pasanen P, . Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis. 2001;155:403–12.
- Toikka JO, Laine H, Ahotupa M, Haapanen A, Viikari JSA, Hartiala JJ, . Increased arterial intima-media thickness and in vivo LDL oxidation in young men with borderline hypertension. Hypertension. 2000;36:929–33.
- Raitakari OT, Toikka JO, Laine H, Ahotupa M, Iida H, Hartiala J, . Reduced myocardial flow reserve relates to increased carotid intima-media thickness in healthy young men. Atherosclerosis. 2001;156:469–75.
- Toikka J, Niemi P, Ahotupa M, Niinikoski H, Viikari J, Rönnemaa T, . Large artery elastic properties in young men: relationships to serum lipoproteins and oxidized low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 1999;19:436–41.
- Linna M, Borg P, Kukkonen-Harjula K, Fogelholm M, Nenonen A, Ahotupa M, . Successful weight maintenance preserves lower levels of oxidized LDL achieved by weight reduction in obese men. Int J Obes. 2007;31:245–53.
- Raitakari M, Ilvonen T, Ahotupa M, Lehtimäki T, Harmoinen A, Suominen P, . Weight reduction with very-low caloric diet and endothelial function in overweight adults. Role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24:124–8.
- Vasankari TJ, Kujala U, Vasankari TM, Ahotupa M. Reduced oxidized LDL levels after a ten-month exercise program. Med Sci Sports Exerc. 1998;30:1496–501.
- Vuorimaa T, Ahotupa M, Irjala K, Vasankari T. Prolonged, acute exercise reduces moderately oxidized LDL in endurance trained men. Int J Sports Med. 2005;26:420–5.
- Jula A, Marniemi J, Huupponen R, Virtanen A, Rastas M, Rönnemaa T. Effects of diet and simvastatin on serum lipids, insulin, and antioxidants in hypercholesterolemic men. JAMA. 2002;287:598–605.
- Janatuinen T, Toikka J, Knuuti J, Ahotupa M, Nuutila P, Rönnemaa T, . The effect of pravastatin treatment on LDL oxidation and myocardial blood flow in young adults with type 1 diabetes. Arterioscler Thromb Vasc Biol. 2004; 24:1303–8.
- Vasankari T, Ahotupa M, Viikari J, Nuotio I, Vuorenmaa T, Strandberg T, . Effects of statin therapy on circulating conjugated dienes, a measure of LDL oxidation. Atherosclerosis. 2005;179:207–9.
- Jenkins DJA, Kendall CWC, Marchie A, Parker TL, Connelly PW, Qian W, . Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine and pulmonary nitric oxide. A randomized, controlled, crossover trial. Circulation. 2002;106:1327–32.
- Vuorimaa T, Ahotupa M, Häkkinen K, Vasankari T. Different hormonal response to continuous and intermittent exercise in middle-distance and marathon runners. Scand J Med Sci Sports. 2008;18:565–72.
- Ahotupa M, Marniemi J, Lehtimäki T, Talvinen K, Raitakari OT, Vasankari T, . Baseline diene conjugation in LDL lipids as a direct measure of in vivo LDL oxidation. Clin Biochem. 1998;31:257–61.
- McAteer MA, Grimsditch DC, Vidgeon-Hart M, Benson GM, Salter AM. Dietary cholesterol reduces lipoprotein lipase activity in the atherosclerosis-susceptible Bio F(1)B hamster. Br J Nutr. 2003;89:341–50.
- Väisänen S, Gävert J, Julkunen A, Voutilainen E, Penttilä I. Contents of apolipoprotein A-I, A-II and B of the human serum fractions for high-density and low-density lipoproteins prepared by common precipitation methods. Scand J Clin Lab Invest. 1992;52:853–62.
- Pilz J, Meineke I, Gleiter CH. Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative. J Chromatogr B Biomed Sci Appl. 2000;742:315–25.
- Lahiri DK, Nurnberger JI Jr. A rapid non-enzymatic method for the preparation of HMW DNA from blood for RFLP studies. Nucleic Acids Res. 1991;19:5444.
- Harangi M, Seres I, Varga Z, Emri G, Szilvassy Z, Paragh G, . Atorvastatin effect on high-density lipoprotein-associated paraoxonase activity and oxidative DNA damage. Eur J Clin Pharmacol. 2004;60:685–91.
- Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–478.
- Marschang P, Götsch C, Kirchmair R, Kaser S, Kähler CM, Patsch JR. Postprandial, but not postabsorptive low density lipoproteins increase the expression of intercellular adhesion molecule-1 in human aortic endothelial cells. Atherosclerosis. 2006;186:101–6.
- Wolff SP, Nourooz-Zadeh J. Hypothesis: UK consumption of dietary lipid hydroperoxides—a possible contributory factor to atherosclerosis. Atherosclerosis. 1996;119:261–3.
- Vasankari T, Fogelholm M, Kukkonen-Harjula K, Nenonen A, Kujala U, Oja P, . Reduced LDL oxidation after weight reduction in obese premenopausal women. Int J Obes Relat Metab Disord. 2001;25:205–11.
- Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, . Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–71.
- Nordestgaard BG, Benn M, Schnohr P, Tybjærg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;297:299–308.
- Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;297:309–16.
- Masella R, Vari R, D'Archivio M, Santangelo C, Scazzocchio B, Maggiorella MT, . Oxidised LDL modulate adipogenesis in 3T3-L1 preadipocytes by affecting the balance between cell proliferation and differentiation. FEBS Lett. 2006;580:2421–9.
- Jialal I, Devaraj S. Antioxidants and atherosclerosis. Don't throw out the baby with the bath water. Circulation. 2003;107: 926–8.
- Marniemi J, Hakala P, Mäki J, Ahotupa M. Partial resistance of low density lipoprotein to oxidation in vivo after increased intake of berries. Nutr Metab Cardiovasc Dis. 2000;10:331–7.
- Christison J, Karjalainen A, Brauman J, Bygrave F, Stocker R. Rapid reduction and removal of HDL- but not LDL-associated cholesteryl ester hydroperoxides by rat liver perfused in situ. Biochem J. 1996;314:739–42.
- Fluiter K, Vietsch H, Biessen EA, Kostner GM, van Berkel TJ, Sattler W. Increased selective uptake in vivo and in vitro of oxidized cholesteryl esters from high-density lipoprotein by rat liver parenchymal cells. Biochem J. 1996;319:471–6.
- Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–5.