Abstract
Hot flushes are complained of by approximately 75% of all postmenopausal women, and hormone therapy (HT) is the most effective way to alleviate them. Hot flushes are characterized by altered vascular function and sympathetic nervous system activity. Hot flushes occurred more often in women attending large, non-randomized observational studies (e.g. Nurses’ Health Study), where HT use protected against cardiovascular disease (CVD). However, they were absent (or mild) in randomized HT trials where HT use was accompanied with an elevated risk for CVD. Hot flushes, if a factor for cardiovascular health, could partly explain the conflict between observational and randomized trials.
Several cross-sectional studies imply that hot flushes are detrimental to the cardiovascular system. However, the data are not uniform, and hot flushes were recalled retrospectively or during HT use. In our prospective study hot flushes were accompanied with a vasodilatory effect during endothelial testing, and this was related to the severity of hot flushes. Night-time hot flushes were followed with transient rises in ambulatory blood pressure (BP). However, no effect of hot flushes on diurnal BP was detected. The use of estradiol showed no harmful effects on endothelial function in women with hot flushes, but in non-flushing women oral, but not transdermal, estradiol led to vasoconstrictive changes. Estradiol complemented with medroxyprogesterone acetate eliminated the vasoconstrictive effect of sole oral estradiol. Thus, both oral and transdermal estradiol are applicable in flushing women, whereas a transdermal route should be favored in non-flushing women if used e.g. for bone protection.
| Abbreviations | ||
| BP | = | blood pressure |
| CEE | = | conjugated equine estrogens |
| CHD | = | coronary heart disease |
| CI | = | confidence interval |
| CVD | = | cardiovascular disease |
| EPT | = | estrogen-progestin therapy |
| ET | = | estrogen-only therapy |
| HERS | = | Heart and Estrogen/progestin Replacement Study |
| HR | = | hazard ratio |
| HT | = | hormone therapy |
| MPA | = | medroxyprogesterone acetate |
| OR | = | odds ratio |
| PWA | = | pulse wave analysis |
| RR | = | risk ratio |
| WHI | = | Women’s Health Initiative |
| WHI-E | = | Women's Health Initiative (Estrogen only) |
| WHI-EP | = | Women's Health Initiative (Estrogen and Progestin) |
Key messages
Hot flushes are a significant determinant for cardiovascular health.
Both oral and transdermal estradiol can be used for alleviating hot flushes, but in non-flushing women transdermal estradiol appears safer than oral estradiol.
Oral estradiol with MPA had neutral vascular effects.
In future trials on cardiovascular health, hot flushes should be considered as a potential confounding factor.
Introduction
Hot flushes, also referred to as hot flashes (in American English) or vasomotor symptoms, occur in up to 75%–80% of all menopausal women (Citation1–3). They adversely affect the quality of life and therefore, estrogen-based hormone therapy (HT), the most effective treatment for hot flushes, has been used in clinical practice for approximately 60 years (Citation4). During this time abundant data on the effects of HT on the occurrence of cardiovascular disease (CVD) have also accumulated (Citation5,Citation6). There are approximately 40 observational studies, which have shown a 30%–50% lower risk of CVD in users of HT. Therefore, up to the late 1990s HT was commonly recommended for the prevention of CVD (Citation7–9). This policy was criticized on the grounds of a lack of randomized trials. Therefore, randomized, placebo-controlled trials, such as the Heart and Estrogen/progestin Replacement Study (HERS) (Citation10,Citation11) and the Women's Health Initiative (WHI) trial (Citation12,Citation13), were undertaken. Surprisingly, HT failed in both secondary and primary prevention of CVD, and, in contrast, the risk of CVD was increased during the initial years of HT use (Citation14,Citation15). Thus, a major conflict was seen, for example, between the results from the largest observational study and randomized, controlled trials ().
Table I. Main outcomes in the largest observational study and randomized, placebo-controlled trials on the cardiovascular effect of hormone therapy.
Numerous plausible explanations for the conflict have been presented (Citation16,Citation17). First, the protective effect of HT against CVD in observational studies may in part be attributed to the ‘healthy woman effect’, i.e. women who chose HT were in general healthier than women who did not use HT. Second, individuals who adhere to any given treatment, whether it is active or placebo, tend to have a more beneficial outcome than those who receive no treatment. Third, the so-called ‘window’ theory proposes that HT may exert harmful vascular effects in arteries of older women, which already possess atherosclerotic plaques. In such blood vessels HT may operate as a thrombogenic agent favoring the rupture of plaques or other thrombogenic events, which ultimately lead to vascular catastrophes (Citation18–20). According to this theory, in younger women with healthier arteries HT may prevent initiation or progress of atherosclerosis through different mechanisms. However, even though this explanation appears solid and is supported by the data of the WHI trial (Citation21–26), it fails to explain why HT appeared to be protective in observational studies also in elderly women (Citation27).
Hot flushes have emerged as an additional explanation for the divergent cardiovascular effects of HT (Citation19,Citation28–33). It should be noted that women with troublesome hot flushes start HT treatment to control their symptoms, and thus it is mainly such women who have been included in HT user groups in observational studies (Citation19). In contrast, hot flushes were mild or absent in the majority of women taking part in the WHI trial (Citation25), where hot flushes were assessed with questionnaires during the preceding 4 weeks. However, in this study women with severe hot flushes were excluded (Citation34). Therefore, HT users in observational studies and in randomized trials differ drastically in their hot flush status. If hot flushes are a factor connected with vascular health, they could perhaps be one explanation for the divergence of cardiovascular data in observational versus randomized studies.
Etiology of hot flushes
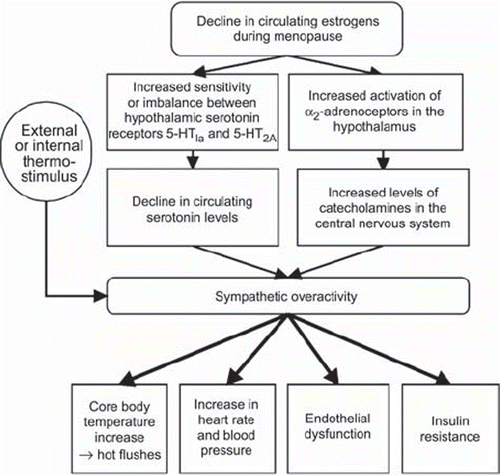
Although the exact etiology of hot flushes is unknown, hypoestrogenism is a key factor (Citation1,Citation3,Citation35,Citation36) (). However, no clear associations between the levels of estrogens or gonadotropins and the presence or severity of hot flushes have been found (Citation36–38). Changes in serotonin, noradrenalin, calcitonin gene-related peptide, and neuropeptide Y also occur in association with hot flushes (Citation39–42) (). Stimulation of the sympathetic nervous system results in a rise in core body temperature. Enhanced sympathetic tone may result, for instance, in altered vascular function (Citation43), changes in blood pressure (BP) (Citation44,Citation45) and lipids (Citation43), and development of insulin resistance (Citation43,Citation46). Hot flushes usually peak within the year of the last menstruation (Citation47) and then subside with advancing age, but approximately 20% of women continue to have hot flushes several years after menopause, and many women have symptoms in their 70s (Citation47–51).
Figure 1. Proposed physiological mechanisms for the initiation of menopausal hot flushes and possible cardiovascular outcomes (modified from (Citation3,Citation33,Citation35,Citation39–42)).
Quantification of hot flushes
A hot flush is a subjective sensation that occurs very individually. Therefore, for research purposes it is mandatory to quantify hot flushes. This can be done, for instance, by measuring dermal temperature increase and/or changes in skin conductance (Citation52,Citation53). These methods are, however, quite laborious and thus inapplicable for large clinical trials. Therefore, for clinical research, vasomotor symptoms can be reliably evaluated with questionnaires in which both the frequency and severity of symptoms are rated (Citation54,Citation55). It is necessary that these questionnaires be completed prospectively and for several days, because hot flushes show a large day-to-day variability (Citation54). Furthermore, a detailed prospective questioning enables estimation of the overall burden of hot flushes.
Cross-sectional studies on hot flushes and markers for cardiovascular risk
There have been several pre-WHI attempts to link hot flushes to various cardiovascular risk factors, such as antioxidant status (Citation56) and BP (Citation57–60). Interpretation of these findings is limited by the small number of study subjects, but, interestingly, simultaneously recorded hot flushes have been shown to be associated with both increased (Citation61,Citation62) and decreased BP (Citation63). After the publication of the WHI results, suggesting cardiovascular harm associated with HT use, a few cross-sectional studies were carried out to study the possible connection between hot flushes and markers for CVD. Several studies revealed signs of an adverse cardiovascular risk profile associated with hot flushes (Citation64–69).
A cross-sectional study with 492 pre- to post-menopausal women showed that hot flushes were associated with significantly lower flow-mediated dilation (implying endothelial dysfunction) and increased calcification in the aorta (odds ratio (OR) 1.63; 95% confidence interval (CI) 1.07–2.49), but not in the coronary arteries (OR 1.31; 95% CI 0.84–2.05) (Citation64). However, it was curious that 11.4% of women with hot flushes and 12.2% of asymptomatic women were using HT, which reduces the possibility to evaluate the impact of pretreatment hot flushes on cardiovascular health. Interestingly, in the subgroup of non-HT users, the aforementioned associations between hot flushes and cardiovascular risk factors were no longer significant. In another study (Citation65) flow-mediated dilation in women with hot flushes (n = 89) was also significantly lower than in the controls (n = 31). In this study, levels of high-density lipoprotein cholesterol were also reduced in women with hot flushes, whereas carotid-intima thickness, BP, and levels of triglycerides and low-density lipoprotein cholesterol were comparable between the groups.
In a large, population-based sample of 5,523 women aged 46–57 years, night sweats were associated with greater elevations in systolic and diastolic BP (1.20 and 0.71 mmHg, respectively) in normotensive women, and this difference was independent of antihypertensive medication (Citation66). Another study by the same group, with 5,857 pre- to postmenopausal women (age 56.3 ± 3.0 years (mean ± standard deviation)), revealed that in women with sweat attacks only, systolic BP was higher in both women with and without antihypertensive medication (Citation67). Furthermore, in this study sweat attacks were associated with higher levels of non-fasting total cholesterol, LDL cholesterol, and triglycerides. Interpretation of these findings is, however, limited due to the facts that vasomotor symptoms were not recorded prospectively before any medication, menopausal status was not controlled for, and lipid analyses were made from non-fasting blood samples. Another recent study, among 343 women with and 260 women without vasomotor symptoms, revealed no association between the presence or severity of hot flushes, and BP (Citation68). Adverse lipid changes in women with hot flushes have also been seen in elderly, osteoporotic women taking part in a raloxifene trial. However, body mass index was also higher in these women (Citation69).
Prospective studies on the impact of hot flushes on cardiovascular health
As reviewed above, some but not all data relate hot flushes to an adverse cardiovascular risk profile. Many previous studies are retrospective, and thus they did not utilize prospective hot flush recording, which may be considered a golden standard (Citation32). Moreover, many confounders were not controlled for. To our knowledge, so far only we have reported results from a trial which involved prospective assessment of hot flushes, followed by study of their relationship to variables reflecting vascular health before and during the use of HT (Citation70–75).
In our trial 150 recently postmenopausal healthy women (age 48–55 years, time since last menstruation 6–36 months) who had either moderate to severe hot flushes (≥ 7/day, symptomatic n = 72) or who were regarded as asymptomatic (no or ≤ 3 mild hot flushes/day, n = 78) were studied at baseline and randomized to receive either transdermal estradiol (1 mg/day), oral estradiol (2 mg/day) with or without medroxyprogesterone acetate (MPA) (5 mg/day), or placebo for 6 months. Hot flushes were prospectively assessed before the initiation of the trial by means of a 2-week diary, and the volunteers kept a record of their vasomotor symptoms throughout the entire trial. Vascular health before and after the 6-month treatment period was assessed by using a number of reliable vascular function markers (Citation70–75).
Assessment of vascular health in our trial
Measurement of arterial stiffness, either as a surrogate end-point or a therapeutic target, has been used in both epidemiological studies and clinical trials (Citation76–80), and it is a valuable tool for the assessment of vascular health in individuals with little or no end-organ disease (Citation78). Endothelial dysfunction is a predisposing factor as regards CVD, and it also contributes to the clinical progression of these diseases (Citation80–83). Pulse wave analysis (PWA) provides a well validated, repeatable, and non- invasive means of assessment of vascular health (Citation84,Citation85). In PWA central aortic waveforms are derived from a series of peripheral pulse waves that are recorded at the radial artery with a tonometer. Such analysis of blood flow in the aorta enables assessment of systemic arterial stiffness (augmentation index) (Citation84,Citation86) and aortic stiffness (time to the return of the reflected wave) (Citation87,Citation88). This method also enables assessment of vascular reactivity and endothelial function by complementing it with nitroglycerin (causes endothelium-independent vasodilation) and salbutamol (causes endothelium-dependent dilation) challenges. In addition, we recorded ambulatory BP for 24 hours (Citation74) and assessed biochemical markers for cardiovascular disease in fasting blood samples (Citation75), both of which are established methods to assess cardiovascular health (Citation89–92). All results were controlled for the possible impact of time since menopause and changes in estradiol levels.
At baseline, PWA assessments showed that increasing severity of hot flushes was accompanied by an arterial vasodilatory feature, as evidenced by prolongations of the times to peak ejection and reflected waveform after the nitroglycerin challenge (Citation70). This indicates a potentially favorable vascular feature in women with hot flushes of different degrees of severity. In addition, severe hot flushes were associated with increases in night-time diastolic BP and heart rate (Citation71). However, these increases were so short that, on the whole, the overall effect of hot flushes on diurnal BP was negligible. However, heart rate variability may be a more sensitive marker of cardiovascular function than heart rate or BP alone, and this variability showed an increase in sympathetic activity and a decrease in parasympathetic activity during a severe hot flush episode (Citation93). We found no association between baseline hot flush status and biochemical markers for CVD, i.e. lipids, apolipoproteins, lipoprotein (a), C-reactive protein, and sex hormone-binding globulin (Citation72).
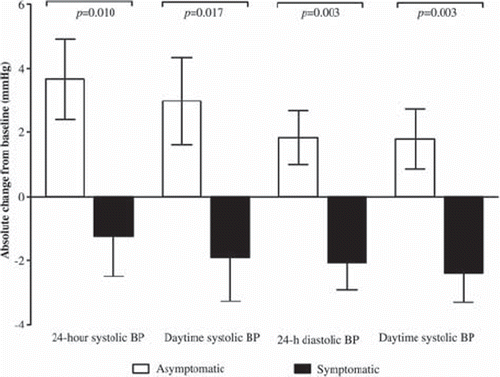
Results from our HT trial show that in asymptomatic women oral estradiol led to decreases in the time to the first systolic peak (13.2%; P = 0.028) and the time to reflected waveform (8.4%; P = 0.018) after a nitroglycerin challenge (Citation73). This potentially unfavorable vasoconstrictive effect, a decrease in vascular reactivity, was not seen in symptomatic women or in connection with the other treatment regimens used (). Additionally, 24-hour systolic (3.7 ± 1.2 mmHg; P = 0.010) and diastolic (1.8 ± 0.8 mmHg; P = 0.003) BPs rose in asymptomatic women receiving oral estradiol, whereas decreases (−1.2 ± 1.2 mmHg and −2.1 ± 0.8 mmHg, respectively) were detected in symptomatic women (). Daytime systolic BP and diastolic BP also increased (3.0 ± 1.3 mmHg, P = 0.017; and 1.8 ± 0.9 mmHg, P = 0.003, respectively) in asymptomatic women with oral estradiol treatment, but the same therapy led to falls (−1.9 ± 1.3 mmHg and −2.4 ± 0.9 mmHg, respectively) in symptomatic women. No such effects were observed as regards transdermal estradiol, or oral estradiol accompanied by MPA (Citation74). The responses of biochemical markers for CVD (i.e. lipids, apolipoproteins, lipoprotein (a), C-reactive protein, and sex hormone-binding globulin) to hormone therapy showed no association with hot flush status (Citation75).
Figure 2. Effect of 6 months of transdermal and oral estradiol, without or with MPA, on vascular reactivity in recently post-menopausal women without hot flushes (modified and reproduced from (Citation73) with permission from Wolter Kluwer Health).
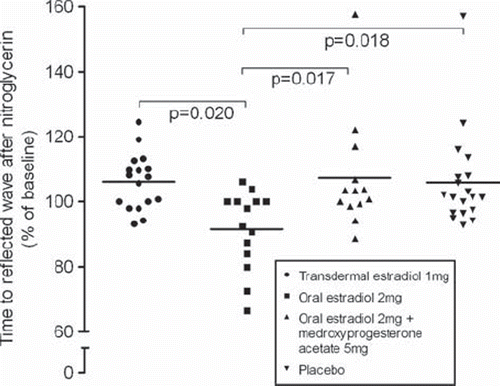
Figure 3. Differential effects of 2 mg oral estradiol on 24-hour and day-time systolic and diastolic blood pressures (BPs) in women with and without hot flushes (from (Citation110), with permission).
As discussed above, our prospective study showed that vasomotor symptoms are a vascular determinant. As limitations to our trial we acknowledge that we studied only lean white women, and thus our results may not be generalized to obese women or women belonging to other ethnic groups. A use of HT for more than 6 months could reveal additional vascular effects, although 3- to 6-month HT treatments have been commonly used in vascular function studies (Citation94). Finally, we studied a limited number of women, and, thus, further studies with larger numbers of subjects are warranted.
Possible mechanisms of action
As reviewed above, menopausal hot flushes may be related to cardiovascular health before and during the use of HT. The mechanism of action can only be speculated upon. However, it seems clear that differences in the concentrations of circulating hormones are an unlikely explanation, because estrogen levels are comparable in women with hot flushes of different degrees of severity (Citation36,Citation70). It is possible that hot flushes become a determinant of cardiovascular health through effects on sympathetic/parasympathetic activity (Citation93). This in turn may regulate the balance of endothelium-derived vasoactive agents (Citation95) or affect insulin sensitivity (Citation96). Based on our findings, hot flushes may reflect potentially more reactive arteries, which respond favorably to exogenous estrogen. This may also be in line with the ‘window’ theory, suggesting detrimental effects of HT in older, more calcified, and less reactive arteries (Citation18). We admit that it is also possible that hot flushes may modify cardiovascular risk through as yet unknown mechanisms, e.g. via the renin-aldosterone axis, or some other significant CVD determinant, and this aspect needs to be further studied. And finally, it should be stated that raloxifene, a selective estrogen receptor modulator, often causes and aggravates hot flushes, although it has shown a cardioprotective effect (Citation97). Raloxifene is a tissue-specific compound; in hypothalamus and breasts it acts as an antiestrogen, but in the cardiovascular system and bone it triggers estrogenic effects (Citation98).
Clinical implications
Based on the present knowledge, hot flushes may imply increased vascular sensitivity to hypoestrogenism, leading to adverse changes in cardiovascular risk factors. Thus, the risk for CVD may rise earlier in women with versus without hot flushes. On the other hand, women with hot flushes show greater vascular sensitivity to exogenous estrogen, and thus these women may benefit from estrogen therapy, not only from the quality of life aspect considered, but perhaps also as regards a reduced risk of CVD. This is in line with the recent scientific statement on HT from the Endocrine Society (Citation99). Regarding HT it is also curious that the route of estrogen administration appears to be a significant determinant for the vascular effects of HT dependent on hot flush status (Citation73,Citation74). Thus, a transdermal route may be advocated for a non-flushing woman if she chooses to use estrogen, e.g. for improving bone or urogenital health. It should be noted that the effect of long-term transdermal HT on BP is still open (Citation100). Moreover, from a vascular point of view, clinicians should not restrict the use of progestin as a part of HT, because the progestin complement, at least as far as MPA is concerned, appears neutral towards the vascular bed (Citation73–75,Citation101). However, the progestin complement of HT seems to be associated with a higher risk of breast cancer, versus estrogen-only therapy (Citation102,Citation103), and, therefore, the impact of progestin cannot be ignored.
Future research
Hot flushes appear to be such a significant vascular determinant that their presence and severity should be carefully recorded in all cardiovascular studies conducted in postmenopausal women. The present data call for detailed reanalyses of older studies, even though such reanalyses may be difficult to carry out due to insufficient data on pre-study hot flush status. Indeed, some attempts have been made. For instance, reanalysis of the WHI data showed that the presence of moderate hot flushes at baseline in women aged 50–59 years was associated with a 14% lower, albeit non-significant, risk of CVD in users of HT (hazard ratio (HR) 0.86; 95% CI 0.44–1.65) (Citation25). Furthermore, hysterectomized women with hot flushes at enrollment had significantly reduced odds as regards coronary calcification (OR 0.66; 95% CI 0.45–0.98) (Citation104). However, another study found an increased risk for aortic calcification, but not for coronary calcification, in elderly women (mean age 62 years) who had a long history of hot flushes (Citation105). Interestingly, data regarding the effects of HT in older women with hot flushes are more divergent. In the WHI study, women aged 70–79 years with moderate to severe hot flushes at the initiation of HT showed an increased risk of coronary heart disease (CHD) during HT use (HR 5.08; 95% CI 2.08–12.40) (Citation25). Reanalyses of the HERS trial data showed that in women over 65 years of age, hot flushes at baseline appeared to be associated with an increased risk of CHD during the first year of HT use (HR 9.01; 95% CI 1.15–70.35) (Citation106), whereas in another reanalysis women with hot flushes and of similar age had a lower all-cause mortality rate (HR 0.72; 95% CI 0.55–0.94) (Citation107). This diversity in the results is interesting and warrants further research, since these trials were not initially designed to assess the impact of hot flushes, and the analyses were carried out retrospectively; hence significant biases may be present. It must be stressed that such reanalyses should be based on reliable hot flush data before the start of HT. We suggest that in all upcoming cardiovascular trials on postmenopausal women, hot flush status should be considered as a potential confounding factor. Furthermore, hot flushes occurring during the use of HT may be a sign of an insufficient dose of estrogen, hyperthyroidism, or some other condition, and, as such, they are not predictive. Finally, future research should also include a focus on older women in their 60s and 70s who still have hot flushes, since the outcome of HT may differ in these women.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health. 2007;10:247–57.
- Freeman EW, Sherif K. Prevalence of hot flushes and night sweats around the world: A systematic review. Climacteric. 2007;10:197–214.
- Sturdee DW. The menopausal hot flush—anything new? Maturitas. 2008;60:42–9.
- Stefanick ML. Estrogens and progestins: Background and history, trends in use, and guidelines and regimens approved by the US food and drug administration. Am J Med. 2005; 118 Suppl 12B:64–73.
- Taskinen MR. Oestrogen replacement therapy and coronary heart disease. Ann Med. 1998;30:443–51.
- Nair GV, Klein KP, Herrington DM. Assessing the role of oestrogen in the prevention of cardiovascular disease. Ann Med. 2001;33:305–12.
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, . Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117: 1016–37.
- Barrett-Connor E. The menopause, hormone replacement, and cardiovascular disease: The epidemiologic evidence. Maturitas. 1996;23:227–34.
- Hu FB, Grodstein F. Postmenopausal hormone therapy and the risk of cardiovascular disease: The epidemiologic evidence. Am J Cardiol. 2002;90:26F–9F.
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, . Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280: 605–13.
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, . Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin replacement study follow-up (HERS II). JAMA. 2002;288: 49–57.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–33.
- Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, . Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34.
- Herrington DM, Klein KP. Randomized clinical trials of hormone replacement therapy for treatment or prevention of cardiovascular disease: A review of the findings. Atherosclerosis. 2003;166:203–12.
- Ylikorkala O. HRT as secondary prevention of cardiovascular disease. Maturitas. 2004;47:315–8.
- Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–50.
- Mikkola TS, Clarkson TB, Notelovitz M. Postmenopausal hormone therapy before and after the women's health initiative study: What consequences? Ann Med. 2004;36:402–13.
- Mikkola TS, Clarkson TB. Estrogen replacement therapy, atherosclerosis, and vascular function. Cardiovasc Res. 2002; 53:605–19.
- Mikkola TS, Ylikorkala O. Hormone therapy and cardiovascular disease—still much to be learnt. Gynecol Endocrinol. 2005;20:116–20.
- Mikkola TS, Clarkson TB. Coronary heart disease and postmenopausal hormone therapy: Conundrum explained by timing? J Womens Health (Larchmt). 2006;15:51–3.
- Grodstein F, Manson JE, Stampfer MJ. Hormone therapy and coronary heart disease: The role of time since menopause and age at hormone initiation. J Womens Health (Larchmt). 2006;15:35–44.
- Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, . Conjugated equine estrogens and coronary heart disease: The women's health initiative. Arch Intern Med. 2006;166:357–65.
- Ouyang P, Michos ED, Karas RH. Hormone replacement therapy and the cardiovascular system lessons learned and unanswered questions. J Am Coll Cardiol. 2006;47: 1741–53.
- Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, . Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–602.
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, . Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77.
- Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, . Observational studies analyzed like randomized experiments: An application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–79.
- Sourander L, Rajala T, Raiha I, Makinen J, Erkkola R, Helenius H. Cardiovascular and cancer morbidity and mortality and sudden cardiac death in postmenopausal women on oestrogen replacement therapy (ERT). Lancet. 1998;352:1965–9.
- van der Schouw YT, Grobbee DE. Menopausal complaints, oestrogens, and heart disease risk: An explanation for discrepant findings on the benefits of post-menopausal hormone therapy. Eur Heart J. 2005;26:1358–61.
- Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–41.
- Allison MA, Manson JE. The complex interplay of vasomotor symptoms, hormone therapy, and cardiovascular risk. Menopause. 2009;16:619–20.
- Andrikoula M, Hardiman P, Prelevic G. Menopausal hot flush: Is it only a nuisance or also a marker of cardiovascular risk? Gynecol Endocrinol. 2009;27:450–4.
- Pinkerton JV, Stovall DW. Is there an association between vasomotor symptoms and both low bone density and cardiovascular risk? Menopause. 2009;16:219–23.
- Gambacciani M, Pepe A. Vasomotor symptoms and cardiovascular risk. Climacteric. 2009;12 Suppl 1:32–5.
- The Women's Health Initiative Study Group. Design of the Women's health initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109.
- Freedman RR. Pathophysiology and treatment of menopausal hot flashes. Semin Reprod Med. 2005;23:117–25.
- Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecol Endocrinol. 2009;25:303–14.
- Overlie I, Moen MH, Holte A, Finset A. Androgens and estrogens in relation to hot flushes during the menopausal transition. Maturitas. 2002;41:69–77.
- Randolph JF Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, . The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–12.
- Barton D, Loprinzi C, Wahner-Roedler D. Hot flashes: Aetiology and management. Drugs Aging. 2001;18:597–606.
- Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–18.
- Whiteman MK, Staropoli CA, Benedict JC, Borgeest C, Flaws JA. Risk factors for hot flashes in midlife women. J Womens Health (Larchmt). 2003;12:459–72.
- Rapkin AJ. Vasomotor symptoms in menopause: Physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196:97–106.
- Pontiroli AE, Pizzocri P, Paroni R, Folli F. Sympathetic overactivity, endothelial dysfunction, inflammation, and metabolic abnormalities cluster in grade III (world health organization) obesity: Reversal through sustained weight loss obtained with laparoscopic adjustable gastric banding. Diabetes Care. 2006;29:2735–8.
- Verdecchia P, Angeli F, Reboldi G. Optimizing the definition of dippers and nondippers: Is a 70–86% full glass empty? J Hypertens. 2008;26:630–2.
- Vongpatanasin W. Autonomic regulation of blood pressure in menopause. Semin Reprod Med. 2009;27:338–45.
- Grassi G, Arenare F, Quarti-Trevano F, Seravalle G, Mancia G. Heart rate, sympathetic cardiovascular influences, and the metabolic syndrome. Prog Cardiovasc Dis. 2009;52:31–7.
- Santoro N. Symptoms of menopause: Hot flushes. Clin Obstet Gynecol. 2008;51:539–48.
- Rödström K, Bengtsson C, Lissner L, Milsom I, Sundh V, Björkelund C. A longitudinal study of the treatment of hot flushes: The population study of women in Gothenburg during a quarter of a century. Menopause. 2002;9:156–61.
- Gold EB, Colvin A, Avis N, Bromberger J, Greendale GA, Powell L, . Longitudinal analysis of the association between vasomotor symptoms and race/ethnicity across the menopausal transition: Study of women's health across the nation. Am J Public Health. 2006;96:1226–35.
- Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Granger AL, Fehnel SE, . Frequency and severity of vasomotor symptoms among peri- and postmenopausal women in the United States. Climacteric. 2008;11:32–43.
- Col NF, Guthrie JR, Politi M, Dennerstein L. Duration of vasomotor symptoms in middle-aged women: A longitudinal study. Menopause. 2009;16:453–7.
- Sievert LL. Variation in sweating patterns: Implications for studies of hot flashes through skin conductance. Menopause. 2007;14:742–51.
- Kronenberg F. Menopausal hot flashes: A review of physiology and biosociocultural perspective on methods of assessment. J Nutr. 2010;140:1380S–5S.
- Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methodologic lessons learned from hot flash studies. J Clin Oncol. 2001;19:4280–90.
- Loprinzi CL, Barton DL. On hot flash mechanism, measurement, and treatment. Menopause. 2009;16:621–3.
- Leal M, Diaz J, Serrano E, Abellan J, Carbonell LF. Hormone replacement therapy for oxidative stress in postmenopausal women with hot flushes. Obstet Gynecol. 2000; 95:804–9.
- Beljic T, Babic D, Marinkovic J, Prelevic GM. Effect of estrogen replacement therapy on cardiac function in postmenopausal women with and without flushes. Gynecol Endocrinol. 1999;13:104–12.
- Brown DE, Sievert LL, Aki SL, Mills PS, Etrata MB, Paopao RN, . Effects of age, ethnicity and menopause on ambulatory blood pressure: Japanese-American and Caucasian school teachers in Hawaii. Am J Hum Biol. 2001;13:486–93.
- James GD, Sievert LL, Flanagan E. Ambulatory blood pressure and heart rate in relation to hot flash experience among women of menopausal age. Ann Hum Biol. 2004; 31:49–58.
- Gerber LM, Sievert LL, Warren K, Pickering TG, Schwartz JE. Hot flashes are associated with increased ambulatory systolic blood pressure. Menopause. 2007;14:308–15.
- Freedman RR, Dinsay R. Clonidine raises the sweating threshold in symptomatic but not in asymptomatic postmenopausal women. Fertil Steril. 2000;74:20–3.
- Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008; 15:290–5.
- Nelesen R, Krohn P, Dimsdale JE. Hot-flash hypotension. N Engl J Med. 2004;351:1577–9.
- Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: Findings from the study of women's health across the nation heart study. Circulation. 2008;118:1234–40.
- Bechlioulis A, Naka KK, Papanikolaou O, Kontostolis E, Kalantaridou SN, Michalis LK. Menopause and hormone therapy: From vascular endothelial function to cardiovascular disease. Hellenic J Cardiol. 2009;50:303–15.
- Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands-van Gent CJ, Samsioe GN, . Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–8.
- Gast GC, Samsioe GN, Grobbee DE, Nilsson PM, van der Schouw YT. Vasomotor symptoms, estradiol levels and cardiovascular risk profile in women. Maturitas. 2010; 66:285–90.
- Gallicchio L, Miller SR, Zacur H, Flaws JA. Hot flashes and blood pressure in midlife women. Maturitas. 2010;65: 69–74.
- Huang AJ, Grady D, Jacoby VL, Blackwell TL, Bauer DC, Sawaya GF. Persistent hot flushes in older postmenopausal women. Arch Intern Med. 2008;168:840–6.
- Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamäki H, Rönnback M, . Evidence for a role of hot flushes in vascular function in recently postmenopausal women. Obstet Gynecol. 2009;113:902–8.
- Tuomikoski P, Haapalahti P, Ylikorkala O, Mikkola TS. Vasomotor hot flushes and 24-hour ambulatory blood pressure in recently post-menopausal women. Ann Med. 2010; 42:216–22.
- Tuomikoski P, Mikkola TS, Hämäläinen E, Tikkanen MJ, Turpeinen U, Ylikorkala O. Biochemical markers for cardiovascular disease in recently postmenopausal women with and without hot flashes. Menopause. 2009;17:145–51.
- Tuomikoski P, Ebert P, Groop PH, Haapalahti P, Hautamäki H, Rönnback M, . Effect of hot flushes on vascular function; A randomized controlled trial. Obstet Gynecol. 2009; 114:777–85.
- Tuomikoski P, Haapalahti P, Sarna S, Ylikorkala O, Mikkola TS. Vasomotor hot flushes and 24-hour ambulatory blood pressure in normotensive women: A placebo-controlled trial on post-menopausal hormone therapy. Ann Med. 2010;42: 334–43.
- Tuomikoski P, Mikkola TS, Tikkanen MJ, Ylikorkala O. Hot flushes and biochemical markers for cardiovascular disease: A randomized trial on hormone therapy. Climacteric. 2010; 13:457–66.
- Davies JI, Struthers AD. Pulse wave analysis and pulse wave velocity: A critical review of their strengths and weaknesses. J Hypertens. 2003;21:463–72.
- Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: Clinical relevance, measurement and treatment. Clin Sci (Lond). 2007;113:157–70.
- Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34:647–51.
- Safar ME. Pulse pressure, arterial stiffness and wave reflections (augmentation index) as cardiovascular risk factors in hypertension. Ther Adv Cardiovasc Dis. 2008;2:13–24.
- Wang X, Keith JC Jr, Struthers AD, Feuerstein GZ. Assessment of arterial stiffness, a translational medicine biomarker system for evaluation of vascular risk. Cardiovasc Ther. 2008;26:214–23.
- Lane HA, Smith JC, Davies JS. Noninvasive assessment of preclinical atherosclerosis. Vasc Health Risk Manag. 2006; 2:19–30.
- Munzel T, Sinning C, Post F, Warnholtz A, Schulz E. Pathophysiology, diagnosis and prognostic implications of endothelial dysfunction. Ann Med. 2008;40:180–96.
- Rachon D, Teede H. Postmenopausal hormone therapy and the risk of venous thromboembolism. Climacteric. 2008;11:273–9.
- Mackenzie IS, Wilkinson IB, Cockcroft JR. Assessment of arterial stiffness in clinical practice. QJM. 2002;95:67–74.
- Wilkinson IB, Hall IR, MacCallum H, Mackenzie IS, McEniery CM, van der Arend BJ, . Pulse-wave analysis: Clinical evaluation of a noninvasive, widely applicable method for assessing endothelial function. Arterioscler Thromb Vasc Biol. 2002;22:147–52.
- O'Rourke MF, Pauca A, Jiang XJ. Pulse wave analysis. Br J Clin Pharmacol. 2001;51:507–22.
- Marchais SJ, Guerin AP, Pannier BM, Levy BI, Safar ME, London GM. Wave reflections and cardiac hypertrophy in chronic uremia. Influence of body size. Hypertension. 1993; 22:876–83.
- Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J Physiol. 2001; 530:541–50.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000; 342:836–43.
- Davison S, Davis SR. New markers for cardiovascular disease risk in women: Impact of endogenous estrogen status and exogenous postmenopausal hormone therapy. J Clin Endocrinol Metab. 2003;88:2470–8.
- Chan DC, Watts GF. Apolipoproteins as markers and managers of coronary risk. QJM. 2006;99:277–87.
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–74.
- Hoikkala H, Haapalahti P, Viitasalo M, Vaananen H, Sovijarvi AR, Ylikorkala O, . Association between vasomotor hot flashes and heart rate variability in recently postmenopausal women. Menopause. 2010;17:315–20.
- Teede HJ. Sex hormones and the cardiovascular system: Effects on arterial function in women. Clin Exp Pharmacol Physiol. 2007;34:672–6.
- Mikkola T, Turunen P, Avela K, Orpana A, Viinikka L, Ylikorkala O. 17 Beta-estradiol stimulates prostacyclin, but not endothelin-1, production in human vascular endothelial cells. J Clin Endocrinol Metab. 1995;80:1832–6.
- Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18.
- Collins P, Mosca L, Geiger MJ, Grady D, Kornitzer M, Amewou-Atisso MG, . Effects of the selective estrogen receptor modulator raloxifene on coronary outcomes in the raloxifene use for the heart trial: Results of subgroup analyses by age and other factors. Circulation. 2009;119: 922–30.
- Cano A, Hermenegildo C, Oviedo P, Tarin JJ. Selective estrogen receptor modulators and risk for coronary heart disease. Climacteric. 2007;10:97–111.
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, . Postmenopausal hormone therapy: An endocrine society scientific statement. J Clin Endocrinol Metab. 2010;95:s1–s66.
- Hemelaar M, van der Mooren MJ, Rad M, Kluft C, Kenemans P. Effects of non-oral postmenopausal hormone therapy on markers of cardiovascular risk: A systematic review. Fertil Steril. 2008;90:642–72.
- Clarkson TB, Appt SE. MPA and postmenopausal coronary artery atherosclerosis revisited. Steroids. 2003;68:941–51.
- Beral V; Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27.
- Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estradiol-progestogen therapy. Obstet Gynecol. 2009;113:65–73.
- Allison MA, Manson JE, Aragaki A, Langer RD, Rossouw J, Curb D, . Vasomotor symptoms and coronary artery calcium in postmenopausal women. Menopause. 2010;17:1136–45.
- Thurston RC, Kuller LH, Edmundowicz D, Matthews KA. History of hot flashes and aortic calcification among postmenopausal women. Menopause. 2010;17:256–61.
- Huang AJ, Sawaya GF, Vittinghoff E, Lin F, Grady D. Hot flushes, coronary heart disease, and hormone therapy in postmenopausal women. Menopause. 2009;16:639–43.
- Svartberg J, von Muhlen D, Kritz-Silverstein D, Barrett-Connor E. Vasomotor symptoms and mortality: The Rancho Bernardo Study. Menopause. 2009;16:888–91.
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–41.
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, . Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. JAMA. 2004; 291:1701–12.
- Tuomikoski P. Postmenopausal hot flushes, vascular health and hormone therapy. Academic Dissertation 2010. Helsinki University Faculty of Medicine. Helsinki: Helsinki University Press; 2010.