Abstract
Aims. We investigated the association between daily sodium intake and each individual component of the metabolic syndrome (MS) as well as the metabolic cluster per se and clarified which of the combinations of MS features is particularly associated with sodium intake.
Methods. A total of 716 subjects from our OPERA (Oulu Project Elucidating Risk of Atherosclerosis) cohort were selected to fill in a food follow-up diary for a 1-week period. The MS was determined using the International Diabetes Federation (IDF) criteria.
Results. Subjects with the MS used more sodium (P < 0.001), less carbohydrate (P < 0.001), less fibre (P = 0.031), and more alcohol (P < 0.001) than those without the MS. High sodium intake was strongly related to elevated BMI (P = 0.003), waist (P < 0.001), and higher fasting blood glucose (P < 0.001). The subjects with the highest sodium intake suffered more often from type 2 diabetes (P = 0.007). Sodium intake was highest in the group where all the MS criteria were present (P < 0.001). High sodium intake was a statistically significant predictor of the MS in logistic regression analysis (P = 0.009). The highest sodium intake was observed in the IDF criteria combination waist + glucose + blood pressure.
Conclusions. These findings suggest that a reduction in sodium intake may be especially beneficial in the treatment of individuals with the MS.
Key words::
| Abbreviations | ||
| ASCVD | = | atherosclerotic cardiovascular disease |
| BMI | = | body mass index |
| BP | = | blood pressure |
| HDL | = | high-density lipoprotein |
| IDF | = | International Diabetes Foundation |
| MS | = | metabolic syndrome |
| RAAS | = | renin–angiotensin–aldosterone system |
| SNS | = | sympathetic nervous system |
| TG | = | triglycerides |
| VLDL | = | very-low-density lipoprotein |
Key messages
The daily intake of sodium is increased in the subjects with the metabolic syndrome (MS); higher amount of daily food consumption does not explain the higher daily sodium intake among our subjects with the MS.
Increased salt intake is related to hypertension, type 2 diabetes, and abdominal obesity.
The highest daily sodium intake was observed in the subjects with elevated plasma glucose and high blood pressure combined with large waist circumference, a dominant factor of the MS by the IDF definition.
Introduction
The metabolic syndrome (MS) is a cluster of risk factors that appear to promote directly the risk of developing atherosclerotic cardiovascular disease (ASCVD) and type 2 diabetes mellitus (Citation1,Citation2). The definition for the metabolic syndrome has varied during the last years, though the different classifications include the same metabolic risk factors: insulin resistance, abdominal obesity, overweight, dyslipidaemia, elevated fasting glucose, and hypertension (Citation3). Insulin resistance is thought to be the main factor, and MS has also been known as the insulin resistance syndrome (Citation4).
Several studies have shown the link between hypertension, a key component of the metabolic syndrome, and salt intake. Reduced intake of dietary sodium lowers blood pressure (BP) and reduces the risk of developing ASCVD (Citation5–7). The blood pressure reduction is not directly linked to the reduction of dietary salt intake. The reduction of BP varies between individuals and is described as sodium sensitivity (Citation8,Citation9). Hypertensive patients that are sodium-sensitive are also relatively insulin-resistant (Citation10). Insulin resistance leads to sodium retention, sympathetic activation, and impairment of endothelial nitric oxide production, which leads to extracellular fluid volume expansion and to increasing BP responses (Citation11,Citation12). Hypertension is quite common in patients with type 2 diabetes, and patients with hypertension are more likely to develop type 2 diabetes (Citation13). In the Finnish 24-h sodium excretion study high sodium intake predicted the risk of type 2 diabetes independently of other risk factors (Citation13).
The Hoffman et al. study suggested that, according to the 24-h urinary excretion study, subjects with the metabolic syndrome might eat approximately 1.5–2 g of salt more than those without the syndrome (Citation14). Recent studies have shown that salt sensitivity of blood pressure is more common in patients with the metabolic syndrome than in those without, and the salt sensitivity of BP is higher the more MS components one has (Citation15–17). In the Chinese study the association between the metabolic syndrome and salt sensitivity remained after participants with hypertension were excluded (Citation15). Two recent studies also report the link between increased urinary sodium excretion, obesity, and high BP (Citation13,Citation14).
In the present study, based on a large population-based cohort of middle-aged subjects, we wanted to measure dietary factors, particularly daily sodium intake, in subjects with and without the metabolic syndrome. Particularly we investigated if there is an association between each individual MS component with daily sodium intake and whether some MS criteria combinations relate to particularly high sodium intake.
Material and methods
Study population
The present study was part of a population-based, epidemiological study addressing the risk factors and disease end-points of atherosclerotic cardiovascular diseases (Oulu Project Evaluating the Risk of Atherosclerosis, OPERA) (Citation18). The study population consisted of 600 hypertensive subjects (300 men and 300 women) and 600 control subjects (300 men and 300 women) living in the city of Oulu. The treated hypertensives, aged 40 ± 59 years at the time of selection, were randomly selected by age stratification (15 subjects yearly) from the Social Insurance Institute register for reimbursement of hypertension medication. The age- and sex-matched controls were randomly selected from the social insurance register maintained by the Social Insurance Institute covering the whole population of the city of Oulu.
The overall study population consisted of 1,200 subjects. Altogether 1,045 subjects (87.8%) participated in the study; 520 of them were hypertensive (262 men and 258 women), and 525 were controls (259 men and 266 women). The medication of the hypertensive patients has been described previously (Citation18). The study was carried out according to the instructions of the Declaration of Helsinki. Informed consent was obtained from each participant. The study was approved by the Ethical Committee of the Faculty of Medicine, University of Oulu.
Data collection
The participating study subjects visited the research laboratory of the Department of Internal Medicine of the University of Oulu, where they underwent a detailed interview and clinical examination by physicians and nurses. During the same visit, the study subjects received food records and instructions on how to complete them (Citation19). They were asked to record all the food they ate for 7 days and to use household measures to evaluate portion sizes. The completed food records were returned to the laboratory. Seventy-nine per cent of the hypertensive subjects and 85% of the control subjects, i.e. 857 of the 1,045 subjects (82%), returned the completed food records. A hundred and forty-one (16%) of the returned food records were not accepted because they were incomplete or inaccurate. Thus, 716 (84%) of the food records were of adequate detail and quality and were accepted as material for the dietary intake study. The data of the whole cohort have been presented by Rantala et al. (Citation18). Alcohol consumption was determined both in an interview by a physician using a validated questionnaire (Citation20) and from the food records. All participants were also asked in detail about their smoking habits, use of medications, medical history, and physical activity, which was assessed using the method described by Grimby (Citation21). Nutrient intakes were calculated from the 7-day food records by the NUTRICA computer program (Social Insurance Institution, Helsinki) using the Finnish nutrient database (Citation22). Anthropometric, blood pressure, and laboratory data were collected as previously described (Citation18).
Definition of the metabolic syndrome
In this study we have used the International Diabetes Foundation (IDF) criteria for the diagnosis of the MS (Citation2). Abdominal obesity, defined as waist circumference ≥94 cm for men of European origin and ≥80 cm for European origin women, plus two of the following factors are required for diagnosis: raised triglycerides (TG) level >1.7 mmol/L or specific treatment for lipid abnormality, reduced high-density lipoprotein (HDL) cholesterol (<0.9 mmol/L in males and <1.1 mmol/L in females) or specific treatment for this lipid abnormality, raised blood pressure (systolic ≥130 or diastolic BP ≥85 mmHg or treatment of previously diagnosed hypertension), raised fasting plasma glucose (≥5.6 mmol/L) or diagnosed type 2 diabetes.
Procedures
Waist circumference was measured to the nearest 0.5 cm with a tape measure midway between the lower rib margin and the iliac crest in light expirium. Blood pressure was measured according to the recommendations of the American Society of Hypertension in a sitting position from the right arm with an oscillometric device (Dinamap® model 18465X, Criticon Ltd, Ascot, UK) after an overnight fast and after a 10–15-minute rest. Three measurements were made at 1-minute intervals, and the means of the last two were used in the analyses.
All the laboratory test samples were obtained after an overnight fast. Plasma was separated from venous blood and stored at 4°C. The venous blood glucose concentration was determined with the glucose dehydrogenase method, and the plasma insulin concentration with the double RIA method (AIA-PACK IRI, Tosoh Corp., Tokyo, Japan). The concentrations of total cholesterol and triglycerides in the plasma and lipoprotein fractions were determined by enzymatic colorimetric methods (kits of Boehringer Diagnostica, Mannheim GmbH, Germany, catalogue nos. 236691 and 701912, respectively) using Kone Specific analyser (Kone Specific, Selective Chemistry Analyser, Kone Instruments, Espoo, Finland). The very-low-density lipoprotein (VLDL) fraction (d < 1.006 g/mL) was separated from plasma by ultracentrifugation in a Kontron TFT 45.6 rotor at 105,000 g and 15°C for 18 h. The VLDL fraction was removed from the ultracentrifuged preparation by tube slicing. The plasma high-density lipoprotein (HDL) cholesterol concentration was determined by mixing 1 mL of the VLDL-free fraction with 25 μL of 2.8% (w/v) heparin and 25 μL of 2 M manganese chloride and by measuring the cholesterol concentration in the supernatant after centrifugation at 1000 g and 4°C for 30 minutes. Insulin sensitivity was assessed using fasting plasma insulin concentrations and a quantitative insulin sensitivity check index (QUICKI = 1/[log (fasting insulin) + log (fasting glucose)]).
Statistical analyses
Statistical analysis was performed by using SPSS version 16.0. To compare the means of the variables measured, analysis of variance (ANOVA) was used. Statistical significances between percentages were measured by using chi-square test. The association between sodium intake and MS was assessed using analysis of covariance (ANCOVA) and logistic regression analyses. P value <0.05 was regarded as significant.
Results
The study population consisted in total of 716 subjects: 342 hypertensive and 374 control cohort subjects. The cohort consisted of 345 males and 371 females, and 35.1% of subjects met the IDF criteria for the MS. In the hypertensive cohort the proportion of MS was 48.2% and in the control cohort 23.0%.
The average daily sodium intake in the subjects with the MS was 1,957 mg(1000 kcal)−1 and in the subjects without the MS 1,848 mg(1000 kcal)−1 (P < 0.001) (). Therefore, the daily amount of sodium intake in the MS subjects was about 6% higher than in subjects without the MS. Measured by the percentage of total energy intake, subjects with the MS used less carbohydrate (P < 0.001) and more protein (P = 0.044). Subjects with the MS used less fibre (P = 0.031) and more alcohol (P < 0.001) than those without MS. The ratio of sodium/potassium intake in our subjects was around 1.
Table I. Average daily intakes of energy and nutrients in subjects with and without the metabolic syndrome (by IDF-criteria).
Daily sodium intake was compared between the different components of the MS. reveals the mean daily sodium intake in relation to the clinical features of the MS. We observed that, of the IDF MS criteria, daily sodium intake was associated with higher waist circumference (P = 0.002), systolic blood pressure (P = 0.003), and plasma glucose (P < 0.001). Daily sodium intake was 9.8% higher in the high systolic blood pressure group, 9.2% higher in the high plasma glucose group, and 6% higher in the higher waist circumference group according to IDF criteria. We made analyses in both genders, and sodium intake was related to IDF MS waist and blood pressure criteria only among females, while the relation between blood glucose criteria and sodium intake was observed in both genders (data not shown).
Table II. Sodium intake (mg(1000 kcal)−1) as means (SD) in subjects positive to individual components of the MS according to IDF criteria and in subjects not having the component of the MS in the whole cohort.
The whole cohort was divided into tertiles according to the daily sodium intakes, and the mean values were adjusted for age, sex, and study group (). A rising trend across the sodium intake tertiles can be seen in the number of male subjects (P < 0.001) and hypertensives (P < 0.001), mean BMI (P < 0.001), waist circumference (P < 0.001), smoking (pack years) (P < 0.001), age (P = 0.002), and fasting glucose (P < 0.001). The percentage of type 2 diabetics (P = 0.007) was the highest among those with the highest sodium intake compared to the subjects with the medium or lowest sodium intake. Fasting glucose was the highest (P < 0.001) among the subjects with the highest sodium intake after adjustment for age, sex, study group, and BMI.
Table III. Characteristics of the study subjects according to sodium intake tertiles.
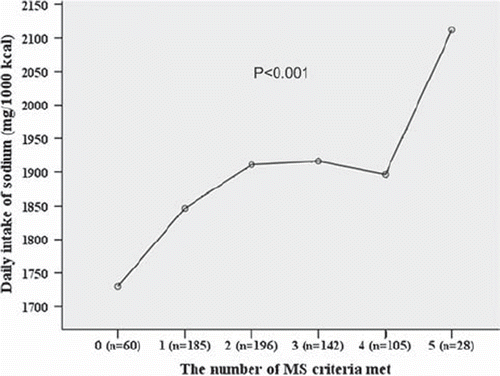
The study subjects were grouped on the basis of the number of components of the MS based on the IDF definition that could be diagnosed. To evaluate the association between the multiplicity of components of the MS and the high sodium intake, dietary sodium intake was compared between the groups. Sodium intake varied in relation to the number of MS IDF criteria met, being highest in the group where all the criteria were present (P < 0.001) (), and when genders were considered separately the latter variation was observed significantly only among females.
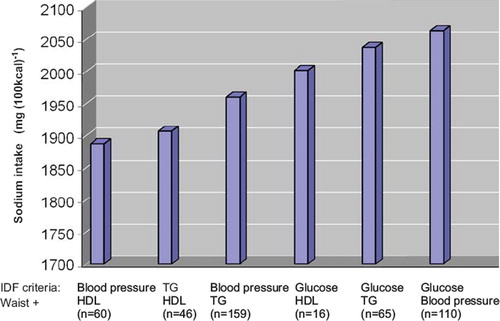
Next we wanted to evaluate whether some MS criteria combinations are related to high sodium intake (). Of the MS feature combinations the highest sodium intake was observed in the groups where elevated blood glucose was included: waist + glucose + HDL, waist + glucose + TG, and especially in the group waist + glucose + blood pressure.
The association between sodium intake and the MS was assessed also by using logistic regression analyses. The effect of the dietary confounding factors significant in univariate analyses were controlled for by adding them into the multivariate models. The following variables were entered into the multivariate models as covariates: alcohol consumption, protein, carbohydrate and fibre intakes, age, and sex. High sodium intake was a statistically significant predictor of MS in logistic regression analysis (P = 0.009) so that the subjects in the third sodium intake tertile were at higher risk of having the MS compared to the subjects in the first tertile (OR (odds ratio) = 1.94; 95% CI 1.23–3.05; P = 0.004). Multivariate model was performed also for both genders separately with the same variables included as above (except sex), and high sodium intake tended to be a significant predictor of the MS in both sexes.
Discussion
The main results of the present study suggest that the daily sodium intake is an independent dietary indicator of the metabolic syndrome. Of the various MS components, high blood glucose seems to show the strongest link with the dietary sodium intake, and manifest type 2 diabetes is about 2.5 times more prevalent in the group with the highest sodium intake.
It has been speculated that the higher daily salt intake in the MS subjects can be explained by the higher amount of daily food consumption (Citation12). Overweight and obese people usually eat more food than normal-weight people. In our study both the subjects with and without the MS received a large amount of total and saturated fat from their diets. The subjects with the MS used less carbohydrate and fibre, and more alcohol and protein than those without the MS. The daily sodium intake was adjusted for total energy intake, and therefore higher amount of daily food consumption does not explain the higher daily sodium intake among our subjects with the MS. Furthermore, although differences in nutrient intake between subjects with and without the MS were adjusted for, high sodium intake was a statistically significant predictor of the MS in logistic regression analysis. The daily sodium intake was higher the more MS components were observed, being highest in the subjects with all five criteria of the MS fulfilled. The same kind of trend has been reported also before: In the Hoffman et al. study the urinary sodium excretion was quite the same in the patients with zero, one, or two components of the metabolic syndrome with NCEP-criteria. Among the patients with three or four components the excretion was higher (Citation14). High sodium excretion was strongly linked with obesity and modestly with higher BP (Citation14). Interestingly, in Hispanics (Citation14) sodium intake was related to blood pressure only in men, but in our study sodium intake was related to IDF MS waist and blood pressure criteria only among females, although the relation between blood glucose and sodium intake was observed in both genders. The reason for these different findings is unknown.
Increased salt intake and hypertension has a documented connection (Citation5–7). Salt sensitivity of blood pressure has been reported to be higher among subjects with the MS (Citation15). As predicted, the daily sodium intake was higher among the subjects with high systolic blood pressure in our study. The study subjects with high sodium intake had also larger waist circumference and higher BMI, which have also been observed in previous studies (Citation13,Citation14). The highest daily sodium intake was observed in the subjects with elevated plasma glucose and high blood pressure combined with large waist circumference, a dominant factor of the MS. Our study is in line with several previous studies: increased salt intake is related to hypertension (Citation5,Citation6,Citation8,Citation15–17), type 2 diabetes (Citation13), and obesity (Citation13,Citation14), which all are independent components of the MS and cardiovascular risk factors. In fact, of the MS feature combinations, the highest sodium intake was observed in the groups where blood glucose was included. Thus our results are in accordance with the Finnish 24-h urinary sodium excretion study, in which the risk of type 2 diabetes was significantly higher in the group with the highest quantities of urinary sodium excretion (Citation13). In our data the prevalence of type 2 diabetics was increased particularly in the group with the highest sodium intake. High BMI and especially abdominal obesity are considered to have a correlation with the type 2 diabetes.
The mechanisms of the connection between salt intake and type 2 diabetes are as yet unknown. Salt intake has been reported to have a connection with insulin sensitivity, the sympathetic nervous system (SNS), and the renin–angiotensin–aldosterone system (RAAS) (Citation23). The effect of salt intake on insulin sensitivity and insulin resistance has been controversial. Some studies have shown an increase in insulin resistance (Citation24–26) followed by a dietary sodium restriction; others found no effect (Citation27). Insulin resistance associated with hyperinsulinaemia might be related to salt sensitivity (Citation10,Citation28–30). Interestingly, an increase in salt sensitivity and decrease in insulin resistance after dietary sodium restriction have been reported (Citation31). Galletti et al. reported that salt-sensitive subjects with hypertension were more insulin-resistant independently of factors such as age and obesity (Citation10). In our study the positive association between insulin resistance and high sodium intake did not, however, persist after the adjustment for BMI. Therefore, sodium intake seems to be strongly linked to obesity-mediated insulin resistance. In the study of Melander et al. insulin sensitivity was not significantly related to salt sensitivity, though increased salt intake and decreased plasma renin activity predicted insulin resistance among the salt-sensitive subjects (Citation32). On the contrary, low salt intake has been reported to activate the RAAS and SNS (Citation33) and thereby to impair insulin signalling (Citation34) and increase insulin resistance (Citation26,Citation34) in some studies.
The Hoffman et al. study suggested that according to the 24-h urinary excretion study, the subjects with the metabolic syndrome might eat approximately 1.5–2 g of salt more than those without the syndrome (Citation14). If the daily sodium intake is converted to daily table salt (NaCl) intake by multiplying it by 2.5, we get an average of 9.1 g of salt for the subjects with the metabolic syndrome and 8.6 g for the subjects without the syndrome. So according to our study the difference is about 0.5 g.
The problem with this kind of study and its results is the reliability and accuracy of the food diary recorded by the subjects. It has been reported that eating habits tend to change during the follow-up: subjects tend to eat less and simpler and healthier food than usually (Citation35). Even up to 18% of under-estimation has been reported concerning the difference in the estimated daily energy intake and the daily energy intake derived from the 24-h food diary (Citation36). In this study the daily salt intake was less than the reported Finnish average at that time, which for men was about 12 g and for women 8 g (Citation37). The most reliable method to estimate the daily sodium intake would have been the measurements of the daily urinary sodium excretion (of the urine), but no such measurements nor data were available. Though the food diaries might under-estimate the daily sodium consumption of the subjects, they show us an important trend and difference in daily sodium intake between the subjects with and without the MS. The same kind of trend, as mentioned before, has been reported before with more accurate urine sodium excretion studies.
The level of caloric intake was rather low among our subjects and did not differ between subjects with and without the MS. The low caloric intake may be due to under-reporting in the food records. It seems that the increase in sodium intake is not due to greater food intake, but rather to greater salty food intake. Salt craving has been described in obesity (Citation38). How could increased salt intake lead to obesity? Increasing intakes of sodium (salt) obligatorily produce a progressive increase in thirst with the concomitant increase in the intake of beverages, which, in turn, is associated with an increase in the intake of calories (Citation6). Considering the other dietary factors, earlier studies have shown that increased intake of dietary total fat may increase the risk of the metabolic syndrome (Citation39), although a low reported energy intake has been associated with the metabolic syndrome in some reports (Citation40). Interestingly, the ratio of sodium/potassium intake in our subjects was around 1, which is a very high ratio. A higher sodium-to-potassium excretion ratio has been associated with increased risk of subsequent cardiovascular disease, with an effect stronger than that of sodium or potassium alone (Citation41).
In conclusion, this study demonstrated that the daily intake of sodium in subjects with the metabolic syndrome is higher than in those without. High sodium intake was strongly related to abdominal obesity, high systolic blood pressure, elevated plasma glucose and type 2 diabetes. As subjects with the metabolic syndrome are at high risk of developing cardiovascular disease, prevention and treatment should be taken seriously. Reduced intake of sodium may be especially beneficial among patients with the metabolic syndrome. Besides various drug treatments the role of dietary interventions cannot be under-estimated in treatment of the MS.
Acknowledgements
This study was supported by the Finnish Foundation for Cardiovascular Research. We acknowledge the excellent technical assistance of Ms Helena Kalliokoski, Ms Saija Kortetjärvi, and Ms Liisa Mannermaa.
Declaration of interest: The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, . Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung and blood Institute Scientific statement. Circulation. 2005;112:2735–52.
- Alberti KG , Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80.
- Lakka H-M, Laaksonen D, Lakka T, Niskanen L, Kumpusalo E, Tuomilehto J, . The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–16.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9.
- Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–90.
- Karppanen H, Mervaala E. Sodium intake and hypertension. Prog Cardiovasc Dis. 2006;49;2:59–75.
- Strazzullo P, D'Elia L, Kandala N-B, Cappuccio F. Salt intake, stroke and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567.
- Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, . Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention. BMJ. 2007;334:885–8.
- Chen J. Sodium sensitivity of blood pressure in Chinese populations. Curr Hypertens Rep. 2010;12:127–34.
- Galletti F, Strazzullo P, Ferrera I, Annuzzi G, Rivellese A, Gatto S, . NaCl sensitivity of essential hypertensive patients is related to insulin resistance. J Hypertens. 1997; 15:1485–91.
- Shimamoto K, Hirata A, Fukuoka M, Higashiura K, Miyazaki Y, Shiiki M, . Insulin sensitivity and the effects of insulin on renal sodium handling and pressor systems in essential hypertensive patients. Hypertension. 1994;23:129–33.
- Grassi R, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell'Oro R, Bolla G, . Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension. 2007;49:535.
- Hu G, Jousilahti P, Peltonen M, Lindström J, Tuomilehto J. Urinary sodium and potassium excretion and the risk of type 2 diabetes: a prospective study in Finland. Diabetologia. 2005;48:1477–83.
- Hoffmann I, Cubeddu L. Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:123–8.
- Chen J, Gu D, Huang J, Rao D, Jaquish C, Hixson J, . Metabolic syndrome and salt sensitivity of blood pressure in non metabolic people in china: a dietary intervention study. Lancet. 2009;373:829–35.
- Uzu T, Kimura G, Yamauchi A, Kanasaki M, Isshiki K, Araki S, . Enhanced sodium sensitivity and disturbed circadian rhythm of blood pressure in essential hypertensive patients with metabolic syndrome. J Hypertens. 2006;24: 1626–32.
- Hoffmann I, Cubeddu L. Increased blood pressure reactivity to dietary salt in patients with the metabolic syndrome. J Hum Hypertens. 2007;21:438–44.
- Rantala AO, Kauma H, Lilja M, Savolainen MJ, Reunanen A, Kesäniemi YA. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Int Med. 1999;245:163–74.
- Silaste ML, Junes R, Rantala AO, Kauma H, Lilja M, Savolainen MJ, . Dietary and other non-pharmacological treatments in patients with drug treated hypertension and control subjects. J Int Med. 2000;247:318–24.
- Kvahari KA, Farbel PD. A profile instrument for the quantification and assessment of alcohol consumption. The Kvahari Alcohol test. J Stud Alcohol. 1978;39:1525–39.
- Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand. 1986;711(suppl):233–7.
- Rastas M, Seppanen R, Knuts LR, Karvetti RL, Varo P. Nutrient composition of foods. Helsinki: Publications of the Social Insurance Institution; 1993.
- Lastra G, Dhuper S, Johnson MS, Sowers JR. Salt, aldosterone and insulin resistance: impact on the cardiovascular system. Nat Rev Cardiol. 2010;7:577–84.
- Fliser D, Fode P, Arnold U, Nowicki M, Kohl B, Ritz E. The effect of dietary salt on insulin sensitivity. Eur J Clin Invest. 1995;25:39–43.
- Petrie J, Morris A, Minamisawa K, Hilditch T, Elliott H, Small E, . Dietary sodium restriction impairs insulin sensitivity in non-insulin dependent diabetes mellitus. J Clin Endocrinol Metab. 1998;83:1552–7.
- Garg R, Williams GH, Hurwitz S, Brown N, Hopkins P, Adler G. Low-salt diet increases insulin resistance in healthy subjects. Metabolism. 2010 Oct 29 (Epub ahead of print).
- Grey A, Braatveldt G, Holdaway I. Moderate dietary salt restriction does not alter insulin resistance or serum lipids in normal men. Am J Hypertens. 1996;9:317–22.
- Giner V, Coca A, de la Sierra A. Increased insulin resistance in salt sensitive essential hypertension. Hypertension. 2001; 15:481–5.
- Sharma AM, Ruland K, Spies KP, Distler A. Salt sensitivity in young normotensive subjects is associated with hyperinsulemic response to oral glucose. J Hypertens. 1991;9:329–35.
- Sharma AM, Schorr U, de la Sierra A. Insulin resistance in young salt-sensitive normotensive subjects. Hypertension. 1993;21:273–9.
- Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC. Effects of sodium intake on insulin sensitivity. Am J Phys Endocrinol Metab. 1993;264:E730–4.
- Melander O, Groop L, Hulthen UL. Effects of salt on insulin sensitivity differs according to gender and degree of salt sensitivity. Hypertension. 2000;35:827–31.
- Perry CG, Palmer T, Cleland SJ, Morton IJ, Salt IP, Petrie JR, . Decreased insulin sensitivity during dietary sodium restriction is not mediated by effects of angiotensin II on insulin action. Clin Science. 2003;105:187–94.
- Lastra G, Manrique C, McFarlane S, Sowers JR. Cardiometabolic syndrome and chronic kidney disease. Curr Diab Rep. 2006;6:207–12.
- Forster JL, Jeffrey RW, VanNatta M, Pirie P. Hypertension prevention trial: do 24-h food records capture usual eating behaviour in a dietary change study? Am J Clin Nutr. 1990;51:253–7.
- Mertz W, Tsui JC, Judd JT, Reiser S, Hallfrisch J, Morris ER, . What are people really eating? The relation between energy intake derived from estimated diet records and intake determined to maintain body weight. Am J Clin Nutr. 1991;54:291–5.
- Kleemola P, Virtanen M, Pietinen P. The 1992 Dietary Survey of Finnish Adults. Kansanterveyslaitoksen julkaisuja B2/1994. Helsinki: Yliopistopaino; 1994.
- Cocores JA, Gold MS. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med Hypotheses. 2009;73:892–9.
- Freire RD, Cardoso MA, Gimeno SG, Ferreira SR; Japanese-Brazilian Diabetes Study Group. Dietary fat is associated with metabolic syndrome in Japanese Brazilians. Diabetes Care. 2005;28:1779–85.
- Buscemi S, Verga S, Donatelli M, D'Orio L, Mattina A, Tranchina MR, . A low reported energy intake is associated with metabolic syndrome. J Endocrinol Invest. 2009;32:538–41.
- Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, .; Trials of Hypertension Prevention Collaborative Research Group. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med. 2009;169:32–40.