Abstract
Dividing people into ‘hypertensives’ and ‘normotensives’ is commonplace but problematic. The relationship between blood pressure and cardiovascular disease is continuous. The Prospective Studies Collaboration analysis shows a continuous straight line dose–response relationship across the entire population down to blood pressure levels of 115 mmHg systolic and 75 mmHg diastolic, the confidence limits on the individual data points being sufficiently narrow to exclude even a minor deviation from a linear relationship. Meta-analysis of randomized controlled trials shows that blood pressure-lowering drugs produce similar proportional reductions in risk of coronary heart disease (CHD) and stroke irrespective of pre-treatment blood pressure, down to levels of 110 mmHg systolic and 70 mmHg diastolic. There are also now sufficient trial data to show a statistically significant risk reduction in ‘normotensive’ people without known vascular disease on entry. The straight line (log-linear) relationship means that the benefit derived from lowering blood pressure is proportional to existing risk, so the decision on whom to treat with blood pressure-lowering drugs should depend on a person's overall absolute risk irrespective of blood pressure. In primary prevention, basing treatment on age alone rather than overall absolute risk entails little loss of efficacy and may be preferred on the basis of simplicity and avoidance of anxiety in telling people they are at elevated risk.
Key words::
Key messages
The relationship between blood pressure and coronary heart disease (CHD) events and stroke is continuous right across the population blood pressure range.
This means that the decision to treat a person with blood pressure-lowering drugs should be based on overall level of risk irrespective of blood pressure.
Age may be used as a surrogate for overall risk in primary prevention for simplicity, with little loss of efficacy.
Categorical distinctions are frequently made in medicine. Breast cancers for example are categorized into oestrogen receptor-positive and oestrogen receptor-negative, because the former respond to tamoxifen or aromatase inhibitors, whereas the latter do not. A similar distinction is made in relation to using blood pressure-lowering drugs to prevent coronary heart disease (CHD) events and stroke: people are categorized into ‘hypertensives’ and ‘normotensives’. This distinction is inappropriate and unhelpful, yet persists despite clear evidence dating back 20 years that the dose–response relationship is continuous (Citation1). Blood pressure contrasts with serum cholesterol in this respect; an analogous categorization of people into ‘hypercholesterolaemics’ and ‘normocholesterolaemics’ was relatively short-lived, and many would now acknowledge that a statin would be effective in lowering a person's risk of CHD irrespective of the pre-treatment cholesterol level. Indeed simvastatin is licensed in the UK to be sold over the counter without measuring cholesterol. But the same is not acknowledged for blood pressure-lowering drugs: the ‘hypertension approach’ persists.
Evidence on the lack of threshold
The Prospective Studies Collaboration meta-analysis of 61 cohort (or prospective observational) studies had sufficient participants (1 million) to determine the dose–response relationship to a high level of precision (Citation2). , adapted from the Prospective Studies Collaboration report (Citation2), shows a straight line relationship between CHD mortality and systolic blood pressure across the entire blood pressure distribution, the confidence limits on the data points being so narrow as to exclude even a minor deviation from a continuous straight line dose–response relationship down to a blood pressure of 115 mmHg systolic. The straight line relationship with mortality plotted on a logarithmic (doubling) scale indicates that a systolic blood pressure reduction of given magnitude from any point on the distribution reduces CHD by a constant proportion of the existing risk: at age 55–64 for example a reduction of 10 mmHg reduces CHD by 25%. The data exclude a threshold. Similar comments apply to the dose–response relationships between systolic blood pressure and stroke and between diastolic pressure and CHD and stroke (Citation2), the straight line relationship extending down to 75 mmHg diastolic.
Figure 1. Dose response relationship between blood pressure and coronary heart disease (CHD) mortality in people aged 55–64 (adapted from the Prospective Studies Collaboration meta-analysis of cohort studies (Citation2)).
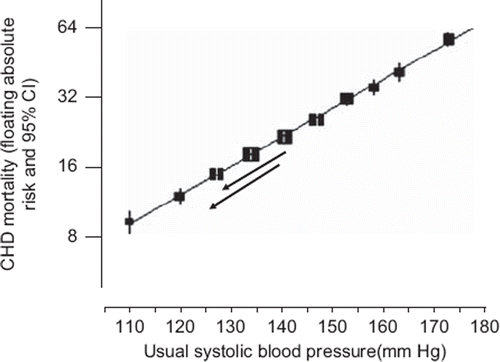
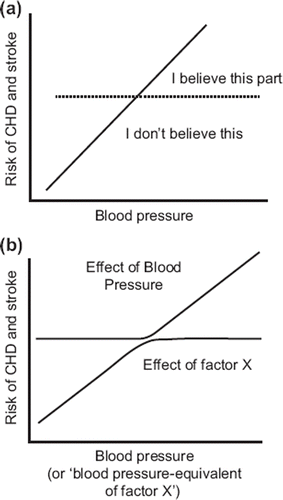
This result is sometimes dismissed on the basis that ‘observational data are unreliable’. But the top part of the dose–response relationship is universally accepted; nobody doubts that treating ‘hypertensives’ prevents CHD and stroke. To accept the upper part of the dose response relationship but attribute the lower part in its entirety to confounding, when the confidence limits establish so perfect a continuous straight line relationship, is implausible (Citation3). One would have to postulate an unknown cardiovascular risk factor (factor X) which is highly correlated with blood pressure, has exactly the same regression slope, and has a dose–response relationship that flattens into a plateau at exactly the same point as that for blood pressure starts to rise from a threshold (). Such an argument can be rejected on the grounds of extreme improbability.
Figure 2. (a) A common response to the evidence from cohort studies showing a continuous straight line dose–response relationship between blood pressure and CHD, and (b) the implausible postulation that must be advanced to justify this negative view (modified from Law & Wald (Citation3)).
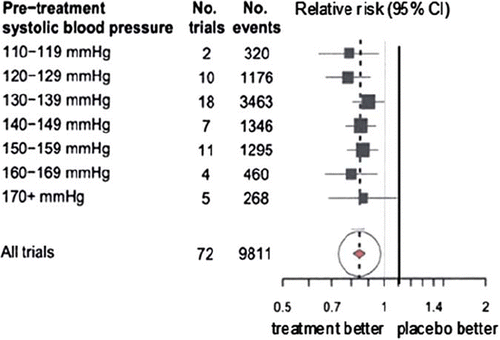
A meta-analysis of 71 randomized trials of blood pressure-lowering drugs published in 2009 confirmed the lack of threshold shown by the cohort study analysis of 2002 (Citation4). summarizes the results, showing the effect of one blood pressure-lowering drug relative to placebo in reducing risk of CHD events with trial participants grouped into those with pre-treatment systolic blood pressures of 110–119 mmHg, 120–129 mmHg, etc., up to 170 + mmHg. The risk reductions are of similar magnitude in each blood pressure group, statistically significant in all but the highest. Even in people with the lowest blood pressure (110–119 mmHg), blood pressure-lowering drugs reduce CHD risk by a similar proportion as in people with higher pressures. Again, there were similar straight line relationships on systolic pressure and stroke and diastolic pressure and CHD and stroke (down to 70 mmHg diastolic). In the meta-analysis the results from the 30 individual trials in which pre-treatment blood pressure was below 140 mmHg (the present ‘hypertension’ cut-off) are entirely consistent with the overall result with no indication of heterogeneity (see web figures in Law et al. (Citation4)).
Figure 3. Summary relative risk estimates of coronary heart disease (CHD) events in randomized controlled trials according to pre-treatment systolic blood pressure (from Law et al. (Citation4); see web figures for individual trial results).
Authorities reluctant to make slight inferences have demanded trial data on participants with both lower blood pressure and no known vascular disease on entry, taking the view that it is already usual clinical practice to prescribe blood pressure-lowering drugs irrespective of the pre-treatment value in patients with coronary heart disease (though not stroke), the term ‘hypertension’ being taken to imply people without vascular disease. This evidence is now available because of recently published trials. summarizes the numbers of participants having a CHD event or stroke, and the corresponding relative risk estimate, in each of the eight published randomized placebo-controlled trials in participants generally without known vascular disease and mean blood pressure in the placebo group below 140 mmHg systolic (and ≤ 80 mmHg diastolic) (Citation5–12). The summary estimate shows a statistically significant risk reduction (P = 0.002).
Figure 4. Meta-analysis of randomized trials of blood pressure-lowering drugs in participants generally without known vascular disease and average pre-treatment blood pressure below 140 mmHg systolic (and ≤ 80 mmHg diastolic), showing the risk of CHD events (myocardial infarction or sudden cardiac death) and stroke combined in treated relative to placebo participants, and the summary estimate from all trials. Participants were selected as having specified disorders in some trials: these were microalbuminuria in one (Citation6), impaired glucose tolerance in two (Citation10,Citation11), type 2 diabetes in two (Citation8,Citation9), and none in three (Citation5,Citation7, Citation12). The proportions of participants known to have vascular disease on entry were 0 in three trials (Citation7,Citation9, Citation11), ≤ 2% in two (Citation6,Citation12), about 20% in one (Citation5), 25% in one (Citation10), and 30% in one (Citation8). Numbers of non-fatal CHD events only (though all strokes) were reported for two of the trials (Citation6,Citation8). Second or subsequent CHD events or strokes in the same participants were not counted, but participants having both a CHD event and a stroke will unavoidably have been counted twice.
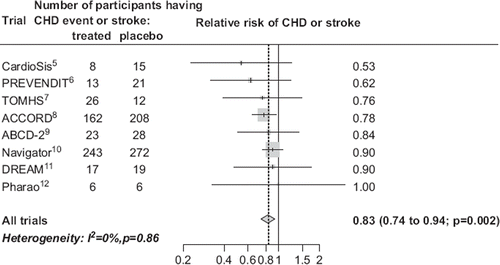
In the numbers of fatal CHD events were unavailable in two trials (Citation6,Citation8) (only non-fatal myocardial infarction was reported), but this was a pre-specified policy (so there is no reason to suspect suppression of unfavourable results). CHD deaths were incorporated into an ‘all cardiovascular deaths’ category, which was not included in the analysis because diseases unrelated to blood pressure were included and will introduce dilution. Also, some participants in the eight trials will have had both a CHD event and a stroke, and these will unavoidably have been counted twice; this is, however, equally likely to have occurred in treated and placebo participants and so will not introduce bias.
Some have accepted that there is no threshold in proportional terms, but argued that in absolute terms the risk reduction is greater from higher blood pressure levels, such that blood pressure-lowering drugs are worthwhile only when pre-treatment pressure is relatively high. This view is mistaken, because a person's blood pressure is a relatively minor determinant of overall risk of CHD or stroke; there are stronger determinants (such as older age or existing atheromatous disease) that cannot necessarily be reversed. In the seven subgroups of participants according to increasing pre-treatment blood pressure in our 2009 meta-analysis summarized in , it happened that the incidence of CHD events in the placebo group was highest (11%) in the group with lowest blood pressure (< 120 mmHg systolic), and second highest (7%) in the group with the next lowest blood pressure (120–129 mmHg); in the other subgroups incidence was lower (3%–6%). Because absolute risk was highest in the group with lowest blood pressure, and proportional risk reduction was similar in all the blood pressure subgroups, absolute risk reduction was highest in those with lowest pre-treatment blood pressure. This is a chance occurrence, dependent on the selection of patients in the different trials, but shows clearly that neither absolute risk nor absolute benefit from blood pressure reduction are necessarily lower at lower blood pressure levels. The effect of treatment is proportional to existing overall risk, whatever the blood pressure.
Implications of the lack of threshold
Acceptance of the lack of a threshold implies a radically different approach to the use of blood pressure-lowering drugs. The term ‘hypertension’ has no utility in the sense that we are all ‘hypertensive’ (the word indicating merely that a person would benefit if their blood pressure was lower than it is). There is no particular value in knowing what a person's blood pressure actually is. (One might argue that there is no proven value in lowering blood pressure from levels around 115 mmHg—there is simply no evidence at these low levels—hence some people may truly be ‘normotensive’, though at older ages such people would account for no more than 1%–2% of the population.) But the present practice of taking multiple blood pressure measurements, to the extent of 24-hour ambulatory blood pressure monitoring, to determine whether a person's average systolic pressure is slightly above or slightly below 140 mmHg is pointless; the arrows in show that both the blood pressure reduction and the risk reduction would be similar either way.
Whether (or when!) a person is advised to take blood pressure-lowering drugs should depend on their absolute risk of CHD or stroke, not their level of blood pressure. The strongest determinant of absolute risk is existing cardiovascular disease, and while patients with coronary artery disease are routinely prescribed beta-blockers and ACE inhibitors, patients with lower blood pressure who have had a stroke are often not prescribed blood pressure-lowering drugs (but they should be!).
In primary prevention, in aiming to prevent first myocardial infarction or stroke (which may be fatal or debilitating), the problem in setting guidelines is that it is not possible to identify a small proportion of the healthy population who will experience the majority of the cardiovascular disease events (Citation3). Blood pressure itself is a poor discriminator, but cardiovascular risk scores are little better. To prevent the majority of first myocardial infarctions and strokes in the population, we need to treat the majority, if not all, people at some stage in their lives.
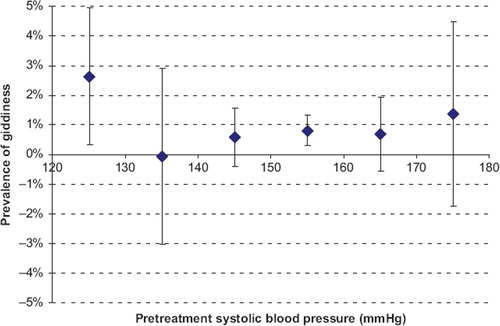
If we are to move towards prescribing blood pressure-lowering drugs for larger numbers of people, we need reassurance that blood pressure-lowering drugs are effective, safe, and inexpensive. This is the case. The major categories of blood pressure-lowering drugs have been widely used for decades, and clinicians are familiar with them. They are off-patent, and, for example, a one-month supply of bendrofluazide, lisinopril, and amlodipine could be available to a health service for as little as 1 euro each. The prevalence of adverse effects is dose-dependent, strikingly so for thiazides and calcium-channel blockers (Citation13), so the drugs should be used in low doses to minimize adverse effects. We have previously advocated using three classes of drug (for example a thiazide, an angiotensin receptor blocker, and a calcium-channel blocker) in combination in half the present standard dose, for greater efficacy (the drugs are additive in their blood pressure-lowering effects) as well as a lower prevalence of adverse effects (Citation13). Meta-analysis of randomized placebo controlled trials has shown that such a combination would cause symptoms in only about 4% of patients (Citation13), reversible on stopping the drug. The drugs are known to be substantially free from serious or non-reversible adverse effects (Citation4,Citation13). Some have voiced concern that prescribing blood pressure-lowering drugs for people below the ‘hypertension’ threshold may cause giddiness, and, with reference to this, shows previously unpublished data from our 2003 meta-analysis of randomized placebo controlled trials of blood pressure-lowering drugs (Citation4). The figure shows that at higher blood pressure levels the drugs themselves (thiazides and calcium-channel blockers especially) cause giddiness in about 1% of people, that in people with systolic blood pressure below 130 mmHg the prevalence of giddiness does not statistically significantly exceed this level of 1%, and that (from the upper confidence limit on the estimate) even if blood pressure reduction below 130 mmHg did cause giddiness the prevalence is unlikely to exceed 5%.
Figure 5. Prevalence of giddiness (placebo adjusted) in meta-analysis of randomized trials of blood pressure-lowering drugs according to pre-treatment of systolic blood pressure (Citation13).
We have previously estimated that three blood pressure-lowering drugs in a low dose combination would reduce the incidence of CHD events by 46% and that of stroke by 63% (Citation4,Citation13). These in combination with a statin (a ‘Polypill’) could be offered to everyone above some relatively young age (say 55) or a relatively low absolute cardiovascular risk in order to prevent the majority of all first CHD events and strokes (Citation14). Age 55 has been proposed as the cut-off on the grounds that the great majority (96%) of deaths from CHD and stroke occur at or above this age (Citation14). Under current practice such a policy would be based on absolute cardiovascular risk, as estimated from risk scores. The advantage of using age alone is that the loss of efficacy is marginal, that virtually everybody would become eligible for treatment at some stage in their life anyway, and that the policy is simple, avoids the cost and doctor's time associated with measuring risk factors (when their discriminatory potential is in any case poor), and avoids the anxiety associated with telling people that their level of cardiovascular risk is elevated. There is no age above which people wishing to take blood pressure-lowering drugs should be denied them on the grounds of possible lack of efficacy, since trial data confirm reduction in CHD and stroke even in people in their 80s (Citation15). The difference between age-based and risk-based policies in the proportion of people in the population who need to be treated at a point in time to attain the same degree of prevention amounts to only a few percentage points and may be justified by the simplicity and avoidance of anxiety associated with the age-based approach (Citation16). The influenza vaccine is an example of a disease prevention policy that is based on age alone.
Acknowledgements
I thank Oliver Redfern for assistance in preparing and Joan Morris for .
Declaration of interest: The author reports no conflicts of interest.
References
- MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, . Blood pressure, stroke, and coronary heart disease. Part 1. Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;355:765–74.
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
- Law MR, Wald NJ. Risk factor thresholds: their existence under scrutiny. BMJ. 2002;324:1570–6.
- Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338: b1665.
- Verdecchia P, Staessen JA, de Simone G, Achilli A, Ganau A, Mureddu G, . Usual versus tight control of systolic blood pressure in non-diabetic patients with hypertension (Cardio-Sis): an open-label randomised trial. Lancet. 2009; 374:525–33.
- Asselbergs FW, Diercks GFH, Hillege HL, van Boven AJ, Janssen WMT, Voors AA, .; for the Prevention of Renal and Vascular Endstage Disease Intervention Trial (PREVENDIT) Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–16.
- Neaton JD, Grimm RH, Prineas RJ, Stamler J, Grandits GA, Elmer PJ, .; for the Treatment of Mild Hypertension Study (TOMHS) Research Group. Treatment of Mild Hypertension Study. JAMA. 1993;270:713–24.
- ; ACCORD (Action to Control Cardiovascular Risk Factors in Diabetes) Study GroupCushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85.
- Schrier RW, Estacio RO, Esler A, Mehler P. Effects of aggressive blood pressure control in normotensive type 2 diabetic patients on albuminuria, retinopathy and strokes (the second Appropriate Blood Pressure Control in Diabetes (ABCD-2) trial). Kidney Int. 2002;61:1086–97.
- ; NAVIGATOR (Nateglinide and Valsartan in Impaired Glucose Tolerance Outcomes Research) Study GroupMcMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, . Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362: 1477–90.
- ; DREAM (Diabetes Reduction Assessment with ramipril and rosiglitazone Medication) InvestigatorsBosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, . Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355:1551–62.
- Luders S, Schrader J, Berger J, Unger T, Zidek W, Bohm M, . The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26:1487–96.
- Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326: 1427–31.
- Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1419–23.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, . Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358: 1887–98.
- Wald N, Simmonds M, Morris J. Screening for future cardiovascular disease using age alone compared with multiple risk factors and age. PLoS One. 2011;6:e18742