Abstract
Purpose. To investigate if an advanced AV search hysteresis (AVSH) algorithm, Ventricular Intrinsic Preference (VIP™), reduces the incidence of ventricular pacing (VP) in sinus node dysfunction (SND) with both intact and compromised AV conduction and with intermittent AV block regardless of the lead positions in the right atria and the ventricle.
Methods. Patients were classified as having intact AV (AVi) conduction if the PR interval was ≤ 210 ms on ECG and 1:1 AV conduction during atrial pacing up to 120 bpm with PR interval ≤ 350 ms. Otherwise the AV conduction was classified as compromised (AVc). Both AVi and AVc patients were randomized to VIP ON or OFF. VIP performed an intrinsic AV conduction search every 30 s for three consecutive atrial cycles with the extension of the sensed and paced AV (SAV/PAV) delays from basic values of 150/200 ms to 300/350 ms. Extended AV intervals were allowed for three cycles when VP occurred before returning to basic AV delays. The primary end-point was %VP at 12 months.
Results. Among 389 patients, 30.1% had intact and 69.9% had compromised AV conduction. The mean %VP at 12 months was 9.6% by VIP compared to 51.8% with standard AV settings in patients with AVi (P < 0.0001) and 28.0% versus 78.9% (P < 0.0001) with AVc. With VIP, excessive %VP among most used lead positions was not seen. Conversely, when VIP was off %VP was low only in patients who had leads in the RA septal–RV septal position (23.0%).
Conclusions. VIP feature reduces VP both in patients with SND and with intermittent heart block regardless of the lead positions in the right atria and the ventricle.
Key messages
A novel and advanced AV search hysteresis algorithm (AVSH) in DDDR pacemaker reduces the incidence of unnecessary ventricular pacing in the majority of sinus node disease patients with both intact and compromised AV conduction and in patients with intermittent AV block.
This is so, regardless of the pacing lead positions in the right atrium and the ventricle.
The avoidance of right ventricular pacing with this AVSH algorithm is not associated with over-long and unphysiologic AV delays.
Introduction
In dual chamber (DDDR) pacing the ventricles should be allowed to be activated by intrinsic atrioventricular (AV) conduction when possible (Citation1,Citation2). However, in both sinus node dysfunction (SND) patients and in patients with intermittent AV block, the use of dual chamber pacemaker (PM), programmed with physiological AV intervals, usually causes unnecessary ventricular pacing (VP) (Citation3–8). Right ventricular (RV) pacing modifies the electrical activation and the contraction pattern of the ventricles, and this has been linked to an increased risk of atrial fibrillation (AF) and heart failure (HF) (Citation9–15). A common perception is that AF and HF hospitalizations show a long-term reduction if %VP is below 40% and are minimized when %VP falls close to 10% (Citation15). On the other hand, substantially prolonged AV conduction reduces ventricular preload, causes mitral regurgitation (Citation16), and is also associated with development of AF. Atrial pacing changes the electrical activation of the atria (Citation17,Citation18). Consequently, induced AV desynchronization with prolonged AV conduction time seems to be disadvantageous when the AV conduction is initially compromised (Citation19). Thus an algorithm in the PM should allow spontaneous ventricular activation when AV conduction is intact or slightly compromised to avoid unnecessary VP. And alternatively, if the AV conduction occasionally or permanently deteriorates, this algorithm should automatically switch the PM to use more physiological AV delays to avoid AV desynchronization.
The purpose of this study was to demonstrate that a novel and advanced AV search hysteresis (AVSH) algorithm, Ventricular Intrinsic Preference (VIP™), reduces the long-term incidence of VP in SND patients with both intact and compromised AV conduction and in patients with intermittent AV block regardless of the lead positions in the right atria and the ventricle.
The focus was on the change of AV conduction and its effect on %VP. Cardiovascular adverse events were also assessed.
Methods
Ethics
The investigation was designed and conducted in accordance with the principles set out in the Declaration of Helsinki. The study protocol was approved by the relevant ethics committees, and all patients gave written informed consent before enrollment and participation in the study.
Study design
This clinical investigation was an international, multicenter, prospective, randomized, parallel, single-blind study designed to evaluate the benefit of the VIP feature in reducing unnecessary VP. A total of 415 patients were recruited between August 2006 and May 2008 in a total of 42 centers in 9 different countries (Belgium, Denmark, Estonia, Finland, Germany, Portugal, Spain, Sweden, and the United Kingdom). The last follow-up visit was conducted on 30 June 2009. Patients were enrolled in the study before post-implant hospital discharge. The randomization was performed 1 month post-implantation. The primary end-point of this multicenter study was %VP at 12 months. These results will be published elsewhere.
Patient selection
Patients with an indication for implantation of a dual-chamber pacemaker, able to attend follow-up visits, not pregnant, over 18 years old, and without permanent or persistent AF or flutter; permanent high-degree AV block, pacemaker replacement, or NYHA class IV heart failure were eligible for study enrollment.
Classification of patients with regard to spontaneous AV conduction
Patients were classified as having intact (AVi) or compromised (AVc) AV conduction at 1 month post-implantation (). Patients were first divided into two groups, based on spontaneous AV conduction:
AVi group: intact AV conduction (PR/AR ≤ 210 ms measured from surface ECG from the beginning of spontaneous or paced atrial activation to the ventricular activation)
AVc group: compromised AV conduction (PR/AR > 210 ms measured from surface ECG)
Secondly the classification was performed based on the results of an AV conduction test. Patients with a PR/AR interval ≤ 210 ms on surface ECG were evaluated further during an AV conduction test. This test ensured that there was 1:1 AV conduction during atrial pacing. The test started at a paced rate of sinus rhythm + 10 bpm. The atrial pacing rate was incremented by 10 bpm until either 120 bpm was reached or Wenckebach behavior was observed. Patients in whom 1:1 AV conduction was lost during the test were classified as AVc. All other patients were classified as AVi, except patients in whom the AR interval was greater than or equal to 350 ms, or who exhibited ventricular pacing at any time during the AV conduction test. This AR interval was measured from the PM IEGM's atrial and ventricular markers.
If the patient was not in sinus rhythm at the time of this evaluation, the patient was excluded from the study. Patients in both of these two groups were then randomized (1:1) to one of two groups defining the use of VIP algorithm:
Control group: VIP algorithm programmed OFF
Treatment group: VIP algorithm programmed ON
In order to evaluate changes in spontaneous AV conduction for patients both in AVi and AVc groups the evaluation of PR/AR intervals from ECG and the AV conduction test was repeated at 12 months.
Pacemakers and leads
All patients enrolled in this study were implanted with a St Jude Medical Victory™ (ST. Jude Medical, Sylmar, CA, USA) or Zephyr dual-chamber pacemaker (model 5810 or 5816), or more recent CE-marked pacemakers with the same study-relevant functionality. CE-marked St Jude Medical bipolar pace/sense right atrial leads were recommended (e.g. Tendril or Isoflex models). CE-marked St Jude Medical bipolar pace/sense right ventricular leads were recommended (e.g. Tendril or Isoflex models). The implanted lead locations in the atria and ventricle were by clinicians’ preference and were reported by the implanter and verified by X-ray. The patients were classified into eight different atrioventricular pacing configurations by their ventricular and atrial lead positions.
Pacemaker settings
All pacemakers were programmed to DDDR mode with a base rate of 60 beats per minute. Maximal tracking rate was 150 bpm, and maximal sensor rate was 150 bpm. The atrial sensitivity was set to bipolar 0.3 mV value. Atrial tachycardia detection rate (ATDR) and also mode switch rate was 200 bpm. Post-ventricular atrial blanking (PVAB) was 125 ms or adapted + 25 ms if FF was present. The AV delays were programmed to sensed AV (SAV) delay 150 ms and paced AV (PAV) delay to 200 ms. Rate-responsive AV delay was not used.
VIP algorithm
Patients were randomized 1:1 to receive either VIP programmed ON or OFF for 11 months. The VIP algorithm is an AVSH algorithm which searches for and promotes intrinsic AV conduction. When the VIP algorithm is activated, the device periodically (every 30 s in the study) extends SAV and the PAV delay by a programmable value (150 ms in the study) for the number of programmed search cycles (three cycles in the study) to search for intrinsic conduction. Additionally, when three consecutive R-waves occur at the programmed SAV or PAV delays, VIP will extend the SAV/PAV delays by the programmed value. If an R-wave is sensed during the extended AV delay, the ventricular pulse is inhibited and the SAV/PAV delays will remain extended until VP occurs. Extended AV intervals are allowed (up to three cycles in the study) when VP occurs before returning to basic AV delays. With VIP algorithm SAV/PAV delays are limited to a maximum of 350 ms, and VIP will not increase the delay beyond the maximum value. For example, when the PAV delay was set to 200 ms in the study, the maximum available VIP setting was 150 ms.
Statistical analysis
For the AVi patients, a total of 39 patients per group (VIP ON or OFF) were needed to detect a difference in mean VP of 38% using an unpaired t test. This test will have 90% power and a two-sided 5% significance level. As the number of patients in the AVi group was assumed to be smaller than the number of patients in the AVc group, 39 patients per group will have a power of > 95%. Continuous variables were expressed as the mean ± SD and range, and the categorical variables were expressed as frequency and percentage. The primary analysis of %VP between VIP ON and OFF within both AVi group and AVc group was based on intention-to-treat (ITT), and it was conducted using non-parametric unpaired t test, Wilcoxon rank sum test, since the assumption for the unpaired t test was violated. The Kruskal–Wallis test, with Monte Carlo estimates on Wilcoxon score, was used for the comparisons between the pacing configuration bearing lowest %VP and the rest pacing configurations, within VIP ON/OFF groups. Bonferroni adjustment on type I error was applied due to the multiple comparisons. Comparisons for other continuous data were implemented by unpaired t test, or equivalent non-parametric test, if the normality assumption was violated. The normality was checked with the aid of normality test, QQ-plot, and the box plot. All categorical characteristics data were compared using chi-square test, or Fisher's exact test, when more than one cell had an expected frequency of less than five. For the relationship between pacing configuration and belonging to AVc/AVi group, the Cochran–Mantel–Haenszel test was implemented for the overall assessment. Chi-square test was further used to perform the pairwise comparison, and the Bonferroni correction was applied on type I error due to the multiple comparison.
Results
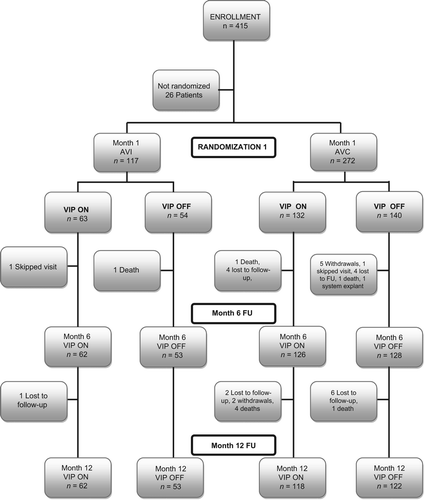
Enrollment, randomization, and patient flow
In total 415 patients were recruited to this study. At the 1 month visit, a total of 389 patients were classified after successful implantation of PM, 117 (30.1%) in the AVi group and 272 (69.9%) in the AVc group. Of the AVc group patients, 106 (39.0%) were classified in this group because of failing to have 1:1 AV conduction in the AV conduction test. Randomization to VIP ON or VIP OFF was performed at month 1. The patient flow is shown in .
Study population
Indications for pacing were: SND in 309 (79%), intermittent AV block in 154 (40%), and a bundle branch block in 79 (20%) of the patients. Intermittent AV block was first-degree in 54 (14%), second-degree in 56 (14%), and third-degree in 45 (12%) of the patients. PM indication was not specified in the remaining three (1.5%) patients. ECG indications of pacing could be various; thus a single patient could have multiple pacing indications. There were differences between the AVi and AVc groups in baseline demographics (). In the AVc group, patients were older and more symptomatic and included more males. The frequencies of different lead positions in the atria were appendage 282 (73.4%), lateral 43 (11.2%), septal 44 (11.5%), and anterior 15 (3.9%) walls and in the ventricle were apex 287 (74.7%), septal wall 95 (24.7%), and other 2 (0.5%). Five patients had missing data on the lead positions. The four lead locations in the right atria and two in the ventricle generated eight different atrioventricular pacing configurations. Cardiovascular adverse events and deaths with both VIP ON and OFF groups are shown in .
Table I. Baseline characteristics of the study population.
Table II. Cardiovascular adverse events and deaths within VIP ON and VIP OFF groups.
%VP for both AVi and AVc group patients with VIP ON or OFF at 12 months
At 1 month the mean ± SD of % ventricular pacing was 53.9 ± 37.2 for all AVi patients and 84.2 ± 24.7 for all AVc patients. The mean and median %VP at 12 months are shown in for all patients in different groups. The VIP feature reduced substantially mean %VP in SND patients within both AVi (P < 0.0001) and AVc (P < 0.0001) groups. At 12 months the absolute reduction in mean %VP was 56.3% with AVc patients and 44.3% with AVi patients, respectively.
Table III. Percentage of ventricular pacing at 12 months for patients in both AVi and AVc groups.
At randomization the mean amount of %AP was 50% (median 53%) and at 12 months 52% (median 54%), respectively. There were no significant differences in %AP between VIP ON or OFF groups.
%VP in regard to different lead locations and pacing configurations and VIP ON/OFF at 12 months
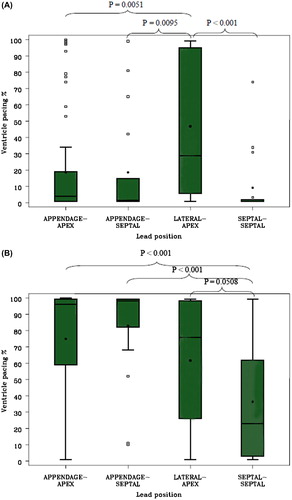
When only comparing patients with VIP ON or OFF the mean %VP in groups with all different pacing configurations with VIP ON was < 40% in 80.1% of patients and < 10% in 62.4% of patients at 12 months. Only four pacing configurations had enough patients from a statistical perspective; therefore, comparisons were done only for the pacing configurations: appendage–apex, appendage–septal, lateral–apex, and septal–septal. There was only one significant difference in the mean %VP among these four different pacing configurations. The mean %VP was significantly lower with patients who had leads in all other positions (9.2%–18.7%) compared to patients with leads in lateral–apical positions (46.8%) (P = 0.0093). Conversely, when the VIP algorithm was disabled the mean %VP in groups with all different pacing configurations was < 40% in 23.4% of patients and < 10% in 11.4% of patients at 12 months. The amount of mean %VP was < 40% only with patients who had leads in the septal–septal position (23.0%). Comparisons of %VP at 12 months in four different pacing configurations with VIP ON and OFF are shown in .
Figure 2. Comparison of %VP at 12 months in four different pacing configurations with box plots of %VP for four pacing configuration within VIP ON group (A) and within VIP OFF group (B). P values are introduced with pacing configurations between lateral–apex compared to other configurations (A) and between septal–septal compared to other pacing configurations (B). Box plot graphs denote 90th and 10th percentiles (whiskers), 75th and 25th percentiles (boxes), medians (horizontal black lines in the boxes), means (black dots), and observations above 90th or below 10th percentile (open square). P values for interactions between two pacing configurations are shown.
Relationship between pacing configuration and belonging to AVc/AVi groups
Patients with leads in RA septal–RV septal positions were more likely to be classified in the AVi group (62.1% in the AVi group) than in the AVc group compared to the patients with leads in appendage–apical (26.3% in the AVi group) (P < 0.0001), appendage–septal (25.0% in the AVi group) (P = 0.001), or lateral –apical (16.2% in the AVi group) (P < 0.0001) positions. shows the frequencies in different pacing configurations and the comparisons between different RA–RV lead positions and relationship to AVc and AVi groups.
Table IV. The frequencies in different lead positions between RA–RV lead positions and AVc and AVi groups. Only the four most frequent were analyzed, because the remaining groups did not have enough patients from a statistical perspective.
The change of PR/AR intervals during the study
The median PR intervals at 1 month were in the AVi group 161 ms ± 27 (median 160, range 80–210) and in the AVc group 220 ms ± 50 (median 220, range 80–440); at 12 months in the AVi group they were 169 ms ± 33 (median 170, range 102–266) and in the AVc group 217 ms ± 75 (median 211, range 125–828). A significant worsening of AV conduction was seen only in the AVi group (P = 0.008). Due to extreme sinus bradycardia, intrinsic AV conduction time could be measured as paced AR interval for 26 (6.7%) patients at 1 month (AVc 256 ms ± 37 (median 242, range 200–336)/AVi mean 169 ms ± 36 (median 183.5, range 94–200)) and for 36 (10.4%) patients at 12 months (AVc 269 ± 45 (median 258, range 180–353) ms/AVi mean 169 ms ± 77 (median 195, range 1.8–253)).
Change in intrinsic conduction assessed by both ECG and the AV conduction test
In total, 261 (78%) patients had no change in classification of intact (AVi) or compromised (AVc) AV conduction. Altogether 39 (12%) patients classified at 1 month in the AVi group were classified as having compromised (AVc) conduction at 12 months, and conversely 33 (10%) of the patients classified at 1 month in the AVc group were classified as having intact AV conduction at 12 months.
In the AV conduction test a high-grade AV block was induced in 106 (39.0%) of the patients at 1 month and in 113 (41.5%) at 12 months in the AVc group. At 12 months AV block was induced in five (4.3%) of the AVi group patients. Atrial paced ventricular sensed AV intervals with pacing rate 10 bpm above intrinsic rate could be measured when there was 1:1 AV conduction. At 1 month the mean interval was 169 ms ± 34 (median 183.5, range 94–200) in AVi patients and 261 ms ± 69 (median 250, range 156–565) in AVc patients; then at 12 months it was 183 ms ± 66 (median 207.5, range 1.8–253) and 260 ms ± 51 (median 250, range 180–400), respectively. Only with the AVi group was worsening in atrial paced intrinsic AV conduction observed, even though the changes were scarce and statistically insignificant in both groups.
The absolute differences between mean spontaneous PR and paced AR intervals were longer with AVc (41 ms) than AVi (8 ms) patients at 1 month. The paced AR intervals at 1 month were also longer with AVc patients (mean 261 ms) than with AVi (mean 169 ms) patients (absolute difference 92 ms).
Discussion
The primary results showed that the use of the VIP algorithm significantly reduces VP in SND patients with both intact and compromised AV conduction and also in patients with intermittent AV block. Furthermore, this is achieved regardless of the lead positions in the right atrium and the ventricle. The mean VP of 9.6% (median 1.0%) in the AVi and 28.0% (median 8.3%) in the AVc group patients with VIP algorithm is highly comparable with other studies addressing the reduction of VP with other advanced AV search hysteresis (AVSH) algorithms (Citation3,Citation6,Citation7).
Prolonged AV conduction is not uncommon also in patients with SND (Citation20). In our study, atrial pacing further prolonged substantially these conduction times with most lead locations. Although the majority of the patients were categorized to have compromised AV conduction, with the VIP algorithm only a minority had an excessive amount of VP. This minority had leads implanted electrically most distantly from each other (RA lateral–RV apical). Conversely, when the VIP algorithm was not used, excessive VP could be avoided only in a minority of the patients. This minority had leads implanted instead most closely to each other (RA septal–RV septal). So it seems that using a PM with VIP algorithm gives the implanting physician more flexibility with choosing alternative pacing lead locations.
In the present study the atrial paced AV delays in patients with impaired AV conduction were notably long, and higher atrial pacing rates furthermore impaired AV conduction. Thus highly effective prevention of VP after atrial pacing appeared unlikely also with patients randomized to the use of VIP algorithm. Since extremely long AV delays or high-grade AV block may adversely influence left ventricular filling and impair cardiac output, the restoration of more physiological AV delays including VP with the VIP algorithm seems reasonable. However, this was not done at the cost of excessive VP, which seems to be more harmful in patients having compromised AV conduction and may lead to more heart failure events and symptoms.
In patients with intact AV conduction, the atrial paced AV intervals rarely exceeded the maximal programmed AV interval value, and thus the %VP with VIP algorithm was very low in our study. Nevertheless, some of these patients deteriorated having compromised AV conduction, some failed 1:1 conduction with higher atrial pacing rates, and the with some atrial paced AV intervals increased substantially during the follow-up. Thus, also in a non-negligible minority (> 10%) of patients with initially intact AV conduction, it can occasionally or permanently deteriorate unfavorably, advocating a need for an advanced AVSH algorithm that constantly adapts the PM to use more physiological AV delays. This is in accordance with the finding in another study where occasional AV block episodes occurred in more than half of the patients with primary SND over just 10 months (Citation21).
An alternative strategy for %VP reduction is the use of algorithms that employ automatic switching between atrial based AAIR and DDDR modes based on continuous search for spontaneous ventricular activation after a sensed or paced atrial event. These algorithms have been shown to be more effective in reducing %VP than standard AVSH algorithms in patients with both intact and compromised AV conduction and with intermittent AV block (Citation6,Citation21,Citation22). However, these algorithms allow excessive (> 350 ms) atrial paced AV intervals, which can be hemodynamically detrimental and are linked to symptomatic episodes, especially during exercise, and increase risk of AF (Citation19,Citation20). It has also been shown that hemodynamics during VP with physiological AV intervals are superior to normal ventricular activation when the atrial paced AV interval exceeds 270 ms (Citation23). Thus the promotion of more physiological AV intervals seems to outweigh the increment of VP.
Several AVSH modifications have recently increased the effectiveness of these algorithms in reducing VP. Only one study has compared standard and another advanced AVSH algorithm in regard to avoiding VP (Citation7). As with our results, this study showed that reduction of VP with advanced AVSH is most pronounced in patients with compromised AV conduction and intermittent AV block. Our results also showed that, although in the majority of patients the AV conduction is quite stable, in a non-negligible minority of patients the AV conduction could either worsen or conversely recover over time. It seems that an AVSH algorithm, like the VIP, with indefinite search for intrinsic AV conduction is useful, and the obligation of the PM physician to check PM event counters and ECGs during follow-up in patients with impaired AV conduction becomes less important.
The rates of mortality, HF, and AF were very low in our study, and there was no sign of benefit or harm for these end-points from the use of the VIP algorithm. These low event rates are similar to other trials of cardiac pacing for SND or only slightly impaired AV conduction in patients with preserved left ventricular function (Citation5–7,Citation24). These findings also support the contention that the reduction of VP has no significant influence on survival with these patients in such a short 1-year follow-up time. In light of recent findings that prolonged intrinsic AV interval is associated with the risk of AF (Citation19,Citation24), and, furthermore, findings that patients having unnecessary VP are also at higher risk of AF (Citation5), it is still, after the present study, not possible to conclude which strategy for VP reduction is clinically superior in relation to AF. In the SAVE PACe trial (Citation5), where AF reduction was achieved with the reduction of unnecessary VP using the MVP algorithm (median %VP 9.1) compared to conventional DDDR pacing, AF reduction could be related not only to the major reduction of VP but also to the use of extremely short and unphysiological AV intervals used with DDDR pacing to ensure 100% VP. However, in the DANPACE trial (Citation24) the excess of AF was restricted to AAIR-paced patients with prolonged AV intervals, and the substantial amount of VP (mean %VP 65) in the DDDR group seemed not to promote AF. Thus in the long term an algorithm, like the VIP as programmed in our study, that promotes intrinsic AV conduction, but not at the cost of over-long AV intervals, could decrease the incidence of AF in these patients. This needs to be investigated in prospective randomized trials with AF as a clinical end-point.
Study limitations
Our study was limited to a single pacemaker manufacturer and AVSH algorithm, impairing the extrapolation of its results to other dual-chamber devices and algorithms. Some of the results derived from relatively small patient subgroups; thus they may not apply to larger patient populations and should be interpreted cautiously. Percentage of ventricular beats that were fusion or pseudofusion was not evaluated from the %VP.
Conclusions
The VIP feature reduces incidence of ventricular pacing in the majority of SND patients with both intact and compromised AV conduction and in patients with intermittent AV block, regardless of the lead positions in the right atrium and the ventricle. The avoidance of RV pacing with VIP is not associated with over-long AV delays.
Declaration of interest: This study was supported by SJM as stated in the manuscript.
References
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA III, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1–e62.
- Vardas PE, Auricchio A, Blanc JJ, Daubert JC, Drexler H, Ector H, et al.;European Society of Cardiology; European Heart Rhythm Association. Guidelines for cardiac pacing and cardiac resynchronization therapy. The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–98.
- Gillis AM, Purerfellner H, Israel CW, Sunthorn H, Kacet S, Anelli-Monti M, et al. Reducing unnecessary right ventricular pacing with the managed ventricular pacing mode in patients with sinus node disease and AV block. Pacing Clin Electrophysiol. 2006;29:697–705.
- Milasinovic G, Sperzel J, Smith T, Mead H, Brandt J, Haisty W. et al. Reduction of RV Pacing by continuous optimization of the AV interval. Pacing Clin Electrophysiol. 2006;29:406–12.
- Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, et al. Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357:1000–8.
- Murakami Y, Tsuboi N, Inden Y, Yoshida Y, Murohara T, Ihara Z, et al. Difference in percentage of ventricular pacing between two algorithms for minimizing ventricular pacing: results of the IDEAL RVP (identify the best algorithm for reducing unnecessary right ventricular pacing) study. Europace. 2010;12:96–102.
- Kolb C, Schmidt R, Dietl JU, Weyerbrock S, Morgenstern M, Fleckenstein M, et al.;PREVENT study group. Reduction of right ventricular pacing with advanced atrioventricular search hysteresis: results of the PREVENT study. Pacing Clin Electrophysiol. 2011;34:975–83.
- Van Oosterhout MF, Prinzen FW, Arts T, Schreuder JJ, Vanagt WY, Cleutjens JP, et al. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation. 1998; 98:588–95.
- Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. 1999;22:1372–7.
- Thambo J, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110:3766–72.
- Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Mortensen PT, Vesterlund T, et al. Long term follow-up of patients from a randomised trial of atrial versus ventricular pacing for sick-sinus syndrome. Lancet. 1997;350:1210–16.
- Lamas GA, Lee KI, Sweeney MO, Silverman R, Leon A, Yee R, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med. 2002;346:1854–62.
- Connolly SJ, Kerr CR, Gent M, Roberts RS, Yusuf S, Gillis AM, et al. Effects of physiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. Canadian Trial of Physiologic Pacing Investigators. N Engl J Med. 2000;342:1385–91.
- Skanes AC, Krahn AD, Yee R, Klein GJ, Conolly SJ, Kerr CR, et al; Canadian Trial of Physiologic Pacing. Progression to chronic fibrillation after pacing: The Canadian Trial of Physiologic Pacing. CTOPP Investigators. J Am Coll Cardiol. 2001;38:167–72.
- Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–7.
- Ishikawa T, Kimura K, Miyazaki N, Tochikubo O, Usui T, Kashiwagi M, et al. Diastolic mitral regurgitation in patients with first-degree atrioventricular block. Pacing Clin Electrophysiol. 1992;15:1927–31.
- Roithinger FX, Abou-Harb M, Pachinger O, Hintringer F. The effect of the atrial pacing site on the total atrial activation time. Pacing Clin Electrophysiol. 2001;24:316–22.
- Strohmer B, Pichler M, Froemmel M, Migschitz M, Hintringer F; ELVIS Study Group. Evaluation of atrial conduction time at various sites of right atrial pacing and influence on atrioventricular delay optimization by surface electrocardiography. Pacing Clin Electrophysiol. 2004;27: 468–74.
- Sweeney M, Ellenbogen KA, Tang ASL, Whellan D, Mortensen PT, Giraldi F, et al; for the Managed Ventricular Pacing Versus VVI 40 Pacing Trial Investigators. Atrial pacing or ventricular backup—only pacing in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:1552–60.
- Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy D, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301:2571–7.
- Stocburger M, Trautman F, Nitardy A, Teetzman MJ, Schade S, Oezlem C, et al. Pacemaker-based analysis of atrioventricular conduction and atrial tachyarrhythmias in patients with primary sinus node dysfunction. Pacing Clin Electrophysiol. 2009;32:604–13.
- Purerfellner H, Brandt J, Israel C, Sheldon T, Johnson J, Tscheliessnigg K, et al. Comparison of two strategies to reduce ventricular pacing in pacemaker patients. Pacing Clin Electrophysiol. 2008;31:167–76.
- Iliev II, Yamachika S, Muta K, Hayano M, Ishimatsu T, Nakao K, et al. Preserving normal ventricular activation versus atrioventricular delay optimization during pacing: the role of intrinsic atrioventricular conduction and pacing rate. Pacing Clin Electrophysiol. 2000;23:74–83.
- Nielsen JC, Thomsen PE, Hojberg S, Moller M, Vesterlund T, Dalsgaard D, et al. A comparison of single-lead atrial pacing with dual-chamber pacing in sick sinus syndrome. Eur Heart J. 2011;32:686–96.