Abstract
It was previously assumed that brown adipose tissue (BAT) is present in humans only for a short period following birth, the time in which mechanisms of generating heat by way of shivering are not yet developed. Although BAT is maximally recruited in early infancy, findings in recent years have led to a new consensus that metabolically active BAT remains present in most children and many adult humans. Evidence to date supports a slow and steady decline in BAT activity throughout life, with the exception of an intriguing spike in the prevalence and volume of BAT around the time of puberty that remains poorly understood. Because BAT activity is more commonly observed in individuals with a lower body mass index, an association seen in both adult and pediatric populations, there is the exciting possibility that BAT is protective against childhood and adult obesity. Indeed, the function and metabolic relevance of human BAT is currently an area of vigorous research. The goal of this review is to summarize what is currently known about changes that occur in BAT during various stages of life, with a particular emphasis on puberty and aging.
Key words::
Throughout human life, there is a gradual loss of brown adipocytes throughout the body.
Puberty, particularly later stages, is associated with transient increases in brown adipose tissue (BAT) that are presently poorly understood but coincident with gains in skeletal muscle.
Age-related changes in BAT correlate with signs of metabolic deterioration, suggesting BAT may be able to provide mechanistic insight into aging and associated metabolic disease.
Adipose tissue is no longer viewed as a simple storage depot, but is instead appreciated as a highly dynamic and metabolically active tissue that plays an essential role in co-ordinated regulation of energy balance. The amount of fat found in the human body is astoundingly broad, ranging from 2% to 70% of total body weight (Citation1), and varies significantly not only across individuals but also within one individual's life-span. This wide range in adiposity largely reflects quantitative differences in white adipose tissue (WAT), the most predominant form of fat. A related subtype of fat called brown adipose tissue (BAT) also varies between people and throughout life, but generally contrary to variations in WAT.
WAT and BAT share a unique capacity for processing lipid, but in the context of metabolism they serve opposing functions. WAT is comprised of white adipocytes specialized for storing large amounts of triglyceride, generally in the form of one large lipid droplet, and secreting various endocrine factors. In contrast, BAT contains cells uniquely designed for lipid oxidation as a means of generating heat. Brown adipocytes have abundant mitochondria and multiple smaller lipid droplets, a form of storage that facilitates lipid–lipase interactions, and are defined by the production of uncoupling protein-1 (UCP1), a mitochondrial pore that, upon stimulation, uncouples adenosine triphosphate generation from oxidative phosphorylation, thereby allowing energy to be dissipated as heat. Generating heat in this way, or non-shivering thermogenesis, is subject to complex co-ordinated regulation in response to cold or diet (reviewed by Cannon and Nedergaard (Citation2)), and involves factors and pathways that include sympathetic innervation, catecholamines, thyroid hormones, growth factors, and natriuretic peptides (Citation3).
It was formerly thought that human BAT is present only during infancy and then disappears shortly after birth as alternative ways of generating heat (e.g. shivering) are established and non-shivering thermogenesis is no longer required for survival (Citation4). However, metabolically active BAT has now been conclusively shown in adult humans (Citation5–9). BAT can be broadly viewed as a diverse set of fat deposits that contain both brown and white adipocytes, the proportion of which differs based on anatomical location (Citation10). More discrete depots of BAT are found in the cervical-supraclavicular region, or between the shoulder-blades, as well as in cervical, axillary, mediastinal, paravertebral, perirenal, and peri-aortic regions (Citation11). However, brown adipocytes are also found interspersed within deposits of WAT, often referred to as beige, brite (brown within white), or recruitable BAT, based on an ability to be activated by cold or sympathetic/adrenergic stimuli (Citation12,Citation13). Anatomically distinct brown adipocytes are all functionally thermogenic (Citation14), but animal data suggest ‘canonical’ BAT shares a precursor with skeletal muscle, whereas beige fat does not (Citation15). For BAT found in the neck region of adult humans, there is currently evidence of both brown and beige features (Citation16–18).
Underscoring the relevance of BAT to humans throughout life, studies suggest BAT activity correlates with favorable metabolic features (Citation19) and is inversely related to body mass index (BMI) (Citation5,Citation6,Citation8,Citation9,Citation20). Similar associations have also been observed in pediatric populations (Citation21), suggesting the intriguing possibility that BAT is protective against childhood and adult obesity and related metabolic dysfunction. While many questions remain regarding the metabolic relevance of human BAT, the aim of this review is to summarize what is currently known about the evolution of BAT throughout the life-cycle, with a particular focus on how BAT changes with puberty and during aging.
Neonates
Human BAT is formed during gestation and maximally recruited at birth, and the transition from fetal to neonatal life represents a rapid and remarkable shift from a quiescent thermoregulatory state to one of maximal rate of heat production (Citation22). Studies have shown that the most metabolically active form of BAT in human infants is the large bilateral supraclavicular depot (Citation23). Indeed, defining features of this depot include extremely rapid activation and a very high capacity for heat production (Citation24). Evidence from autopsy studies suggests additional more deeply situated BAT in the mediastinal and abdominal regions (Citation25), as well as in the axillary and deep cervical regions (Citation26) of human infants. BAT can account for as much as 5% of total body weight at birth (Citation27), and is a critical means of thermoregulation, as newborn humans do not yet possess the ability to shiver.
Highlighting the degree to which thermogenesis is activated in newborn infants, pronounced depletion of BAT lipid stores is apparent within just a few days of life (Citation25). Further, when newborn infants are exposed to cooler temperatures, BAT likely mediates a rapid doubling of oxygen consumption rates (Citation28). The capacity for stimulated BAT thermogenesis is highly dependent on UCP1 content, and BAT from pre-term and stillborn infants has significantly less UCP1 than older infants, consistent with premature infants having reduced thermogenic capacity and stillborn infants not having been exposed to cold temperatures (Citation26). BAT activity remains extremely high for the first 8 weeks of life, and subsequently declines into childhood (Citation29).
Childhood and adolescence
As with infancy, adolescent BAT is located predominantly in the supraclavicular region (Citation21), but whereas BAT is present in virtually every infant, metabolically active BAT is detectable at room temperature in 20%–75% of children and adolescents, with a similar frequency observed in young boys and young girls (Citation21,Citation30–32). While this highlights an age-dependent decline in BAT activity that begins very early in life (), emerging evidence suggests there may be a transient increase in the prevalence and volume of BAT around the time of puberty (Citation21,Citation30,Citation32), adding provocative support to BAT having a function outside of being a substitute for undeveloped shivering mechanisms.
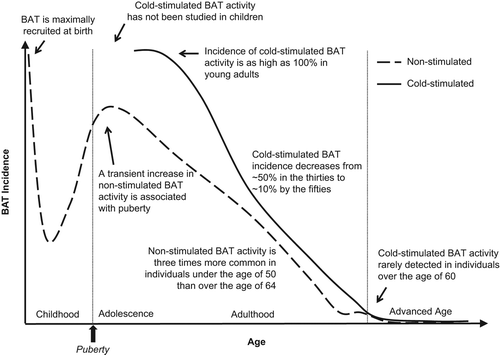
Initial evidence of a puberty-associated increase in BAT activity was reported by Gelfand and colleagues in 2005. These investigators retrospectively examined BAT incidence using [18F]2-fluoro-2-deoxyglucose (FDG)-based positron emission tomography (FDG-PET) scans performed in pediatric cancer patients and found that children over the age of 10 years had a higher incidence of BAT compared to those under the age of 10 years (Citation30). In a similar retrospective study by Drubach et al., analysis of FDG-PET scans from oncology patients between the ages of 1 and 21 years revealed an increase in BAT incidence from childhood to adolescence, with the highest incidence of BAT observed around the age of 13 years (Citation21). These studies collectively support a putative increase in adolescent BAT activity around the time of puberty. Because both studies analyzed BAT in subjects at room temperature, observations may suggest that puberty positively modulates basal BAT activity or reduces the degree of cold required to activate BAT.
In 2012, Gilsanz and colleagues analyzed BAT at different stages of sexual development and found that pediatric BAT prevalence and volume are highly dependent on age and level of sexual maturity, and, conversely, independent of BMI, sex, or season. In this retrospective study, BAT was examined in disease-free patients who underwent FDG-PET combined with X-ray computed tomography (PET/CT) as a follow-up for a previous malignancy (Citation32). Although the presence or absence of malignant disease reportedly has no effect on the ability to detect BAT by PET/CT (Citation33–35), it is worth noting that this study detected BAT in 59% of the tumor-free patients (Citation32), whereas in 2011 a prevalence of 42% was observed using scans from many of the same pediatric patients at a time in which tumors were present (Citation31). Within the overall incidence of 59%, Gilsanz et al. found that prevalence in pre-pubertal children (Tanner stage 1) was only 15%, whereas in older subjects (Tanner stages 2–5) it was 75%. They report a positive association between BAT incidence and degree of sexual development in all five Tanner stages of puberty, but find the relationship is most predominant during Tanner stages 4–5, or late puberty. This study similarly showed that the volume of BAT increases with age and degree of sexual development and particularly during Tanner stages 4–5 (Citation32). Taken together, emerging correlative evidence supports the intriguing possibility of a pubertal increase in BAT activity at room temperature ().
From retrospective FDG-PET/CT studies done by Gilsanz et al. in 2011, it was reported that BAT-positive boys and girls have on average 30%–50% greater muscle volume compared to pediatric patients without identifiable BAT (Citation31). The authors extended these findings in 2012, showing skeletal muscle and BAT display correlative increases in volume throughout puberty, with both preferentially increasing later during sexual development (Tanner stages 4–5) (Citation32). In another study, investigators found that pediatric BAT volume is positively associated with appendicular bone mass, independent of other bone determinants such as height, weight, and gender, in disease-free patients with detectable BAT (Citation36). Collectively, these findings suggest there is a transient increase in BAT activity during adolescence that is coincident with sexual maturation and associated musculoskeletal development (Citation32).
Although findings to date are based exclusively on retrospective analyses of clinically warranted FDG-PET/CT scans, and therefore lack supporting functional data in human or relevant animal models, synchronized growth of BAT and skeletal muscle during puberty is consistent with reports that these two tissues are developmentally related (Citation15). Further, many systemic hormones that promote pubertal musculoskeletal development, such as growth hormone, gonadal sex steroids, insulin, or insulin-like growth factor 1 (IGF-1) (Citation37), may also favor growth of BAT (Citation38–40).
Another interesting possibility is that BAT and skeletal muscle modulate the activity of one another more directly. As described recently by Ponrartana et al., circumstances associated with counterintuitive integrity of musculoskeletal development share a defining feature of considerable BAT abundance. Such contexts include the absence of mechanical loading in relatively motionless infants that simultaneously progress through a period of rapid musculoskeletal development, and hibernating mammals that maintain muscle mass while remaining completely immobile for nearly 7 months (Citation41). These observations imply more direct lines of communication between BAT and the musculoskeletal system.
Indeed, there is accumulating evidence that BAT may have an endocrine function analogous to that of WAT (Citation42). Recent findings suggest fibroblast growth factor 21 is a cold-stimulated human brown adipokine (Citation43,Citation44), and additional BAT-derived factors, such as IL-6 (Citation45) and IGF-1 (Citation46), are implicated in amelioration of diabetes that results from BAT transplantation in rodents. BAT could therefore theoretically have an anabolic effect on skeletal muscle by secreting IGF-1 or other relevant factors.
Alternatively, the musculoskeletal system might release factors that modulate the development of BAT. Circulating factors secreted by skeletal muscle include irisin, which has been shown to positively regulate brown adipogenesis (Citation47). There is presently very little known about irisin during adolescence, but one report suggests circulating concentrations of irisin are higher in young girls compared to young boys and negatively associated with fasting blood glucose levels (Citation48). It would be interesting in future studies to determine whether circulating concentrations of irisin vary according to stage of sexual maturity.
Although there are presently more questions than answers regarding adolescent BAT, in part because invasive aspects of detection have precluded the possibility of prospective study to date, recent observations suggest puberty may favorably influence BAT activity. The observation that occurrence of BAT during adolescence is inversely associated with fat accumulation during subsequent years (Citation49) supports metabolic relevance, but whether the putative increases in BAT observed during this period meaningfully alter energy expenditure or other metabolic indices is unknown. As less invasive and more sensitive imaging techniques emerge, a more detailed understanding of this transient re-emergence of BAT coincident with puberty should be revealed. This will be important, as a better understanding of mechanisms that govern endogenous BAT reactivation could facilitate efforts to reactivate BAT pharmaceutically as a strategy for treating obesity and metabolic disease.
Adulthood and aging
The anatomical distribution of BAT in adults is similar to that observed in adolescents (Citation21), with the most prominent and metabolically active depot in the cervical-supraclavicular region and smaller depots located in the axillary, mediastinal, paravertebral, epicardial, and abdominal areas (reviewed in (Citation24)). The transition from adolescence into adulthood is associated with reduced ability to detect unstimulated, basal BAT activity, as studies collectively suggest ∼5% of adults have detectable BAT under thermoneutral conditions, whereas BAT is detected in 15%–50% of patients under the age of 18 under similar conditions () (Citation50–52). As with pediatric populations, the presence of unstimulated adult BAT is associated with favorable metabolic features (Citation19).
Table I. Brown adipose tissue prevalence in pediatric and adult populations.
Because BAT is activated by cold temperatures, the true prevalence of BAT cannot be derived from retrospective analysis of FDG-PET/CT scans performed at room temperature. Cold-stimulation studies have not been performed in children, but evidence suggests prevalence of cold-stimulated BAT in adults could be as high as ∼100% (Citation7), at least for individuals under the age of 40 years (Citation6). Interestingly, BAT prevalence observed in pediatric patients at 24°C (Citation53) approaches the range observed in adults at 21°C (). More detailed studies are needed, but this may suggest that, at a given temperature, a higher degree of BAT activation occurs in children compared to adults.
In addition to attenuated activity, the capacity for heat production in supraclavicular BAT, as well as the rapidity with which it can be activated, declines into adulthood, perhaps reflecting BAT that becomes increasingly ‘beige’ as a function of age (Citation54). Indeed, autopsy studies performed in 1972 showed that the widest distribution and greatest activation of brown fat occurs in the first decade of life and that many, if not all, anatomical fat deposits subsequently lose brown-like characteristics over the next eight decades (Citation55). Consistent with the primary function of BAT being to generate heat, these studies suggest peripheral depots (e.g. interscapular) are the first to lose brown fat with age, whereas more deeply situated depots, such as those surrounding the kidneys or aorta, retain brown-like features as far as the eighth decade of life. Interestingly, loss of BAT may plateau around the sixth decade of life, followed by a subsequent decline into later decades (Citation55), which may help explain why elderly individuals lose the ability to regulate body temperature effectively (Citation56) and are often unable to tolerate cold temperatures (Citation57).
Findings from a number of more recent studies using FDG-PET/CT to visualize BAT in live subjects () further support a marked decline in BAT activity with age (Citation5,Citation9,Citation20,Citation33,Citation35,Citation58,Citation59). In 2009, Cypess and colleagues retrospectively analyzed ∼2000 adult scans that were clinically warranted for various reasons and found that non-stimulated BAT could be detected in individuals under the age of 50 years three times more often than in individuals over the age of 64 years (Citation5). Similar analyses using FDG-PET/CT scans performed for cancer staging showed that, within BAT-positive cohorts, age is associated with reduced intensity of BAT and fewer BAT-positive depots, with evidence of a particularly, steep decline in the later years (Citation33,Citation35). Studies also suggest age-associated loss of non-stimulated BAT activity may be more pronounced in males (Citation20) and overweight individuals (Citation5). Although a variety of uncontrolled factors such as clothing and climate might affect the ability to detect BAT activity at room temperature, general trends for non-stimulated BAT incidence as a function of age are summarized in (dashed line).
There is also evidence that cold-stimulated BAT activity is attenuated with age (Citation9,Citation59). Saito and colleagues used FDG-PET/CT to visualize BAT in healthy volunteers under warm and cold conditions and found cold-activated BAT could be detected in 52% of younger subjects (aged 23–35 years) compared to only 8% of older subjects (aged 38–65 years) (Citation9). Similarly, Yoneshiro et al. observed cold-induced activity of supraclavicular BAT in more than half of subjects in their 20s, but only 7% of subjects in their 50s and 60s () (Citation59). These studies collectively suggest cold-stimulated BAT activity may drop precipitously around middle age and become undetectable with advancing age. Cold-stimulated BAT incidence as a function of age is summarized in (solid line), with caveats that include limited or absent cold-stimulation studies performed in elderly and pediatric subjects, respectively.
Importantly, because FDG-PET/CT studies quantify only BAT that can be activated, findings to date support age-related deficits in BAT activity, which may or may not reflect changes in mass. Functional deficits are consistent with findings from rodents, as the interscapular BAT depot in rats becomes enlarged with age, likely due to white adipocyte infiltration, but this is coincident with a significant decline in UCP1 activity (Citation60) and loss of function (Citation61). A striking age-dependent loss of UCP1 is also observed in rodent subcutaneous WAT (Citation62), consistent with the morphological evidence derived from human autopsy studies that looked at fat deposits throughout the body (Citation55). Likewise, fat found in human bone marrow, sometimes referred to as yellow adipose tissue (YAT) based on an increased number of mitochondria and other BAT-like features, loses BAT-like characteristics with age (Citation63). It therefore seems that many if not all forms of BAT are gradually transitioned towards WAT with increasing age.
Underlying mechanisms that promote loss of BAT with age remain elusive. Although mechanisms are likely to be complex and synergistic, a few of the pathways that have been implicated are discussed briefly below. Data regarding human BAT are currently limited and largely correlative. While caution should be used before extrapolating findings to humans, drawing on the animal literature will be incorporated in an effort to provide what are likely to be relevant mechanistic clues.
Because diminished proliferative capacity and cellular senescence often accompany aging, age-related loss of BAT might occur as a result of changes to the brown adipocyte precursor pools. Evidence currently supports the presence of precursors during aging, at least for some period of time, as brown adipocyte progenitors have been successfully isolated from donors as old as 78 years (Citation64). However, the possibility of functional deficits cannot be ruled out. A detailed characterization of human brown adipocyte-specific progenitor function during aging remains an important future study, but age-dependent declines in the capacity of white adipocyte precursors to proliferate and differentiate are well documented (reviewed in (Citation65)). Further, rodent BAT displays a diminished proliferative response with age, and, while mechanisms are not fully elucidated, this has been attributed to altered availability of trophic factors (Citation66).
Indeed, there are a number of age-related changes in the somatotropic and gonadotropic axes that could directly or indirectly lead to BAT dysfunction. Aging is associated with a decline in the production of growth hormone, estrogens, and androgens, all of which may positively modulate activity of BAT (Citation38–40). Moreover, a significant correlation between thermogenic parameters of BAT during aging and serum levels of sex steroid hormones has been shown in rodents (Citation67). Changes in concentrations of estrogens might be particularly relevant, as high expression of estrogen receptor in human fetal BAT (Citation68) suggests tissue-level regulation.
Based on reports suggesting glucocorticoids have negative effects (Citation69) whereas estrogens and androgens may have positive effects on BAT (Citation38,Citation39), Nedergaard and Cannon hypothesized an ‘endocrine switch’ whereby a decrease in sex hormones in late adulthood leads to disinhibited actions of glucocorticoids and, consequently, loss of BAT activity (Citation70). Additional hormones implicated in the beneficial actions of estrogens on BAT include thyroid hormone (Citation67) and leptin, based on evidence that estradiol is required for hypothalamic actions of leptin (Citation71) that include regulation of BAT (Citation72). The importance of testosterone to BAT function is less clear. Androgen-deficient mice are obese, and while there is evidence of decreased UCP1 expression (Citation39) there are also reports that BAT is unaltered in these mice (Citation73), as well as orchidectomized rats (Citation74). Further, when testosterone is administered to male mice, BAT mass, protein content, and mitochondrial mass are reportedly unchanged, suggesting testosterone modulates body weight without altering BAT thermogenesis (Citation75).
Thyroid hormone is an appreciated regulator of thermogenesis (Citation76), and human aging is associated with a decrease in serum triiodothyronine (Citation77) and reduced conversion of inactive thyroxine (T4) to active triiodothyronine (T3) (Citation78), a reaction that requires type II iodothyronine deiodinase (DIO2). Likewise, thermogenic parameters in rodent BAT correlate with circulating levels of T3 during aging (Citation67), and age-dependent loss of DIO2 expression is evident in murine WAT (Citation62). Observations such as regulation of DIO2 activity by estrogen (Citation79) suggest significant cross-talk between the thyroid and gonadotropic axes in modulating BAT activity, which could mean that age-dependent alterations to these two systems are collectively more detrimental.
Finally, one of the most critical regulatory systems controlling the activity of BAT is the sympathetic nervous system (SNS). The SNS controls local catecholamine concentrations, and catecholamines in turn act on adrenergic receptors located on the surface of adipocytes to modulate brown adipogenesis and thermogenesis (Citation2). A decline in SNS input and/or impaired downstream signaling could therefore result in a marked decrease in BAT activity. Current evidence argues against an age-dependent loss of SNS stimulation, as a number of studies show that basal as well as stress-induced plasma noradrenaline levels are increased, not decreased, with age in humans (Citation80,Citation81). Likewise, neural recordings done in rodents suggest sympathetic signaling is elevated with age (Citation82), and findings from BAT norepinephrine turnover rate studies do not support blunted sympathetic stimulation to BAT as a cause of declining thermogenic capacity with age (Citation83).
What seems clear from rodent studies is that, although neural signals are intact, thermogenesis increases to a smaller degree with a given sympathetic stimulus with increasing age (Citation84,Citation85). This may be due in part to a decrease in adrenergic receptor density in interscapular BAT, as this is observed in rats with age (Citation86). Age-related down-regulation of adrenergic receptor expression is also observed in rodent subcutaneous WAT, in conjunction with pronounced loss of UCP1 expression and additional intracellular changes, such as enhanced expression of enzymes that degrade catecholamines, that collectively support an age-dependent loss of local adrenergic tone (Citation62).
Consistent with lower basal adrenergic tone, humans with low room temperature BAT activity can still recruit BAT in the cold (Citation87). Similarly, older rodents display a marked loss of beige fat under normal conditions but maintain the ability to recruit beige fat in response exogenous adrenergic stimuli (Citation62). These findings collectively implicate age-related resistance to actions of catecholamines as a contributing factor in associated loss of BAT activity. However, there are also findings that suggest non-SNS pathways may play a major role in BAT activation, including lack of BAT activation in humans in response to pharmacologic activation of the SNS (Citation88) and evidence that fat cells can directly sense cold temperature and activate thermogenesis (Citation89).
In the end, aging is associated with a ubiquitous decline in BAT activity, but details remain obscure, and particularly those specific to loss of human BAT activity. A few candidate mechanisms have been discussed, but it is likely that a myriad of changes coincident with aging may collectively promote a definitive loss of BAT function over time. Because loss of BAT is associated with various pathologies that include metabolic dysfunction and aberrant temperature regulation, a more thorough understanding of mechanisms that facilitate loss as a consequence of age could be beneficial to future efforts to prolong human health span. It will also be critical to elucidate whether there is a point in which BAT is no longer dormant, but instead absent, as humans age, so that age groups most likely to benefit from future therapies that target BAT can be identified.
BAT and age-related metabolic disease
There is a clear correlation between risk factors for metabolic disease (i.e. BMI, age) and reduced BAT activity (Citation5,Citation6,Citation8,Citation9,Citation20), and loss of metabolically active BAT is associated with accumulation of body fat that occurs specifically during aging (Citation59). However, while WAT and BAT are consistently inversely related (Citation5,Citation6,Citation8,Citation9), whether decreases in BAT cause subsequent adiposity or increases in WAT lead to dysfunctional BAT remains less clear.
In the former scenario, a decline in BAT activity that is otherwise programmed into aging could reduce energy expenditure and thereby provide a basis for over-accumulation of WAT. Evidence for a programmed loss of brown adipocytes is derived from rodent caloric restriction studies that show weight-gain is not required for age-associated loss of subcutaneous beige fat (Citation62). The notion that activation of human BAT may increase energy expenditure is supported by extrapolated calculations (Citation7), as well as more recent studies showing that activation of human BAT via cold exposure is associated with measurable increases in energy expenditure (Citation87,Citation90,Citation91) and reduced adiposity (Citation87). However, there are also other reports suggesting that cold-simulated thermogenesis in human BAT accounts for as little as 15 kcal/day, which argues little contribution of BAT to whole-body energy expenditure (Citation92).
Alternatively, it is possible that an increase in WAT precedes an involution of BAT. Increased WAT stores provide more insulation, which might dictate a decrease in heat-generating mechanisms such as non-shivering thermogenesis. Further, because loss of BAT activity is associated with the infiltration of white adipocytes, ectopic accumulation of WAT within BAT could itself lead to dysfunction, as detrimental effects of ectopic lipid are well-documented in other metabolic tissues such as liver and skeletal muscle (Citation93). Supporting the importance of changes in WAT, a reduction in WAT accumulation through caloric restriction can attenuate age-dependent loss of mitochondrial function in canonical rodent BAT (Citation61).
Importantly, BAT activity is favorably associated with a variety of metabolic parameters outside of BMI such as insulin secretion (Citation94), glucoregulation (Citation35,Citation95), lipoprotein metabolism (Citation96), triglyceride clearance (Citation97), and circulating concentrations of HDL (Citation19), all of which are conversely unfavorably associated with aging. Indeed, accumulating evidence suggests BAT is protective against metabolic disease. In mice, subcutaneous beige fat decreases dramatically with age, and this decline is associated with age-dependent loss of glucose homeostasis (Citation62,Citation98). Similarly, obese humans with diabetes have lower levels of UCP1 in subcutaneous WAT than similarly obese but diabetes-free individuals (Citation99), suggesting subcutaneous beige fat is protective against obesity-associated metabolic disease. More recently it was shown that transplanting donor BAT into the visceral cavity of recipient mice prevents impaired glucose tolerance from developing in response to high-fat diet (Citation45). Remarkably, protection was also observed with autonomous BAT transplantation, which may support the design of therapeutics that promote browning of WAT as a means of treating age-associated metabolic disease. Whether detrimental changes in BAT function that accompany aging are directly responsible for associated systemic metabolic dysfunction remains unclear, but a more thorough understanding of the metabolic relevance of BAT is likely to provide insight into underlying mechanisms that link aging and associated metabolic dysfunction.
Concluding remarks
Contrary to previous belief, BAT does not simply involute following birth, as it has now been unequivocally observed in both children and adults. BAT is found in similar anatomical locations, and activity correlates with favorable metabolic features at all stages of life. Although BAT activity generally declines with age, there is one exception around the time of puberty (Citation21). The transient spike in BAT activity during adolescence is poorly understood, but may provide a unique opportunity to study endogenous mechanisms that regulate BAT development. Natural reactivation of BAT also highlights plasticity that adds significant support to its relevance as a therapeutic target. It would be interesting in future studies to learn whether this reactivation is specific to depots in the neck and shoulder region or if this effect extends to brown adipocytes in other areas, such as subcutaneous WAT.
Considerable changes in BAT biology are observed during puberty and with aging, albeit in opposing directions. Likewise, these two contexts are associated with a number of opposing endocrine fluctuations and, in particular, contrary changes in the production of sex hormones. These observations collectively implicate a regulatory role for sex hormones in changes in BAT throughout the life-cycle. Based on current evidence, it is possible that steroid hormones such as estrogen act directly on BAT and/or mediate effects indirectly through other hormones (e.g. leptin), or other tissues, such as skeletal muscle.
During puberty, the volume of BAT and skeletal muscle increase in parallel, and, conversely, a decline in the function of both BAT and skeletal muscle is observed with advancing age. Skeletal muscle produces factors such as irisin that can positively influence BAT development, and BAT produces factors such as IGF-1 that can favor growth of skeletal muscle; interestingly, in both contexts the secreted factors are linked favorably with glucose homeostasis (Citation46,Citation48). However, the degree to which age-related changes in BAT are mechanistically linked to changes related to the size and/or function of skeletal muscle (or vice versa), and how this might relate to insulin sensitivity, which often precipitously declines with age, are presently unknown but warrant future study.
Finally, although great strides have been made, the presence of BAT is likely still underestimated due to detection limitations. FDG-PET/CT is most commonly employed, but this method detects only metabolically active BAT (Citation100), which may or may not reflect the total amount of BAT present. While a decline in the activity of BAT with age is clear, an important unanswered question from a therapeutic point of view is if and when BAT becomes completely absent with age. As detection methods improve, understanding the precise timing of the steep decline in BAT activity in later years and whether or not a small fraction of BAT remains dormant with advancing age will be important questions to address in future studies. Likewise, the question of whether or not BAT can be reactivated in elderly individuals as it can in younger adults (Citation87,Citation91), and the degree to which this could be used to treat age-associated metabolic disease, will need to be determined in the future.
Declaration of interest: The authors report no conflicts of interest.
References
- Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid cellular turnover in adipose tissue. PLoS One. 2011;6:e17637.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359.
- Whittle A. Searching for ways to switch on brown fat: are we getting warmer?J Mol Endocrinol. 2012;49:R79–87.
- Enerback S. Human brown adipose tissue. Cell Metab. 2010;11: 248–52.
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8.
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25.
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–20.
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–31.
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10:24–36.
- Lecoultre V, Ravussin E. Brown adipose tissue and aging. Curr Opin Clin Nutr Metab Care. 2011;14:1–6.
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–64.
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–53.
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep. 2013;5:1196–203.
- Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007;104:4401–6.
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–76.
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS One. 2012;7:e49452.
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013; 19:635–9.
- Zhang Q, Ye H, Miao Q, Zhang Z, Wang Y, Zhu X, et al. Differences in the metabolic status of healthy adults with and without active brown adipose tissue. Wien Klin Wochenschrift. 2013 Nov;125:687–95.
- Pfannenberg C, Werner MK, Ripkens S, Stef I, Deckert A, Schmadl M, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59:1789–93.
- Drubach LA, Palmer EL 3rd, Connolly LP, Baker A, Zurakowski D, Cypess AM. Pediatric brown adipose tissue: detection, epidemiology, and differences from adults. J Pediatr. 2011;159:939–44.
- Symonds ME, Bird JA, Clarke L, Gate JJ, Lomax MA. Nutrition, temperature and homeostasis during perinatal development. Exp Physiol. 1995;80:907–40.
- Hu HH, Tovar JP, Pavlova Z, Smith ML, Gilsanz V. Unequivocal identification of brown adipose tissue in a human infant. J Magn Reson Imaging. 2012;35:938–42.
- Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–90.
- Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91:223–34.
- Lean ME, James WP, Jennings G, Trayhurn P. Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond). 1986;71:291–7.
- Carter BW, Schucany WG. Brown adipose tissue in a newborn. Proc (Bayl Univ Med Cent). 2008;21:328–30.
- Dawkins MJ, Scopes JW. Non-shivering thermogenesis and brown adipose tissue in the human new-born infant. Nature. 1965; 206:201–2.
- Emery JL, Dinsdale F. Structure of periadrenal brown fat in childhood in both expected and cot deaths. Arch Dis Child. 1978;53:154–8.
- Gelfand MJ, O’Hara SM, Curtwright LA, Maclean JR. Pre-medication to block [(18)F]FDG uptake in the brown adipose tissue of pediatric and adolescent patients. Pediatr Radiol. 2005;35:984–90.
- Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 2011;158:722–6.
- Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–9 e1.
- Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–7.
- Lee P, Greenfield JR, Ho KK, Fulham MJ. A critical appraisal of the prevalence and metabolic significance of brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2010;299:E601–6.
- Persichetti A, Sciuto R, Rea S, Basciani S, Lubrano C, Mariani S, et al. Prevalence, mass, and glucose-uptake activity of (1)(8)F-FDG-detected brown adipose tissue in humans living in a temperate zone of Italy. PLoS One. 2013;8:e63391.
- Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97:2693–8.
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–46.
- Rodriguez-Cuenca S, Monjo M, Gianotti M, Proenza AM, Roca P. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17beta-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292:E340–6.
- Yanase T, Fan W, Kyoya K, Min L, Takayanagi R, Kato S, et al. Androgens and metabolic syndrome: lessons from androgen receptor knock out (ARKO) mice. J Steroid Biochem Mol Biol. 2008; 109:254–7.
- Hioki C, Yoshida T, Kogure A, Takakura Y, Umekawa T, Yoshioka K, et al. Effects of growth hormone (GH) on mRNA levels of uncoupling proteins 1, 2, and 3 in brown and white adipose tissues and skeletal muscle in obese mice. Horm Metab Res. 2004;36:607–13.
- Ponrartana S, Hu HH, Gilsanz V. On the relevance of brown adipose tissue in children. Ann N Y Acad Sci. 2013;1302:24–9.
- Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue?Am J Physiol Endocrinol Metab. 2013;305:E567–72.
- Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab. 2013; 98:E98–102.
- Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. Int J Obes (Lond). 2014;38:170–6.
- Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–23.
- Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–82.
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
- Al-Daghri NM, Alkharfy KM, Rahman S, Amer OE, Vinodson B, Sabico S, et al. Irisin as a predictor of glucose metabolism in children: sexually dimorphic effects. Eur J Clin Invest. 2013 Nov 5. [Epub ahead of print]
- Chalfant JS, Smith ML, Hu HH, Dorey FJ, Goodarzian F, Fu CH, et al. Inverse association between brown adipose tissue activation and white adipose tissue accumulation in successfully treated pediatric malignancy. Am J Clin Nutr. 2012;95:1144–9.
- Cohade C, Mourtzikos KA, Wahl RL.”USA-Fat”: prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J Nucl Med. 2003;44:1267–70.
- Truong MT, Erasmus JJ, Munden RF, Marom EM, Sabloff BS, Gladish GW, et al. Focal FDG uptake in mediastinal brown fat mimicking malignancy: a potential pitfall resolved on PET/CT. AJR Am J Roentgenol. 2004;183:1127–32.
- Yeung HW, Grewal RK, Gonen M, Schoder H, Larson SM. Patterns of (18)F-FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med. 2003;44:1789–96.
- Zukotynski KA, Fahey FH, Laffin S, Davis R, Treves ST, Grant FD, et al. Seasonal variation in the effect of constant ambient temperature of 24 degrees C in reducing FDG uptake by brown adipose tissue in children. Eur J Nucl Med Mol Imaging. 2010;37:1854–60.
- Symonds ME, Henderson K, Elvidge L, Bosman C, Sharkey D, Perkins AC, et al. Thermal imaging to assess age-related changes of skin temperature within the supraclavicular region co-locating with brown adipose tissue in healthy children. J Pediatr. 2012; 161:892–8.
- Heaton JM. The distribution of brown adipose tissue in the human. J Anat. 1972;112(Pt 1):35–9.
- McDonald RB, Day C, Carlson K, Stern JS, Horwitz BA. Effect of age and gender on thermoregulation. Am J Physiol. 1989;257(4 Pt 2):R700–4.
- Horvath SM, Radcliffe CE, Hutt BK, Spurr GB. Metabolic responses of old people to a cold environment. J Appl Physiol. 1955;8:145–8.
- Stefan N, Pfannenberg C, Haring HU. The importance of brown adipose tissue. N Engl J Med. 2009;361:416–17; author reply 418–21.
- Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity. 2011;19:1755–60.
- Gabaldon AM, Florez-Duquet ML, Hamilton JS, McDonald RB, Horwitz BA. Effects of age and gender on brown fat and skeletal muscle metabolic responses to cold in F344 rats. Am J Physiol. 1995; 268(4 Pt 2):R931–41.
- Valle A, Guevara R, Garcia-Palmer FJ, Roca P, Oliver J. Caloric restriction retards the age-related decline in mitochondrial function of brown adipose tissue. Rejuvenation Research. 2008;11:597–604.
- Rogers NH, Landa A, Park S, Smith RG. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging Cell. 2012;11:1074–83.
- Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50:546–52.
- Crisan M, Casteilla L, Lehr L, Carmona M, Paoloni-Giacobino A, Yap S, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008;26:2425–33.
- Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66–75.
- Florez-Duquet M, McDonald RB. Cold-induced thermoregulation and biological aging. Physiol Rev. 1998;78:339–58.
- Valle A, Santandreu FM, Garcia-Palmer FJ, Roca P, Oliver J. The serum levels of 17beta-estradiol, progesterone and triiodothyronine correlate with brown adipose tissue thermogenic parameters during aging. Cell Physiol Biochem. 2008;22:337–46.
- Velickovic K, Cvoro A, Srdic B, Stokic E, Markelic M, Golic I, et al. Expression and subcellular localization of estrogen receptors alpha and beta in human fetal brown adipose tissue. J Clin Endocrinol Metab. 2014;99:151–9.
- Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol Cell Endocrinol. 2000;165:7–15.
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–72.
- Pantaleao TU, Mousovich F, Rosenthal D, Padron AS, Carvalho DP, da Costa VM. Effect of serum estradiol and leptin levels on thyroid function, food intake and body weight gain in female Wistar rats. Steroids. 2010;75:638–42.
- Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–84.
- Sato T, Matsumoto T, Yamada T, Watanabe T, Kawano H, Kato S. Late onset of obesity in male androgen receptor-deficient (AR KO) mice. Biochem Biophys Res Commun. 2003;300:167–71.
- Rivest S, Landry J, Richard D. Effect of exercise training on energy balance of orchidectomized rats. Am J Physiol. 1989;257(3 Pt 2):R550–5.
- Abelenda M, Nava MP, Fernandez A, Puerta ML. Brown adipose tissue thermogenesis in testosterone-treated rats. Acta Endocrinol (Copenh). 1992;126:434–7.
- Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–92.
- Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The aging thyroid. Endocr Rev. 1995;16:686–715.
- Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–80.
- Wasco EC, Martinez E, Grant KS, St Germain EA, St Germain DL, Galton VA. Determinants of iodothyronine deiodinase activities in rodent uterus. Endocrinology. 2003;144:4253–61.
- Ziegler MG, Lake CR, Kopin IJ. Plasma noradrenaline increases with age. Nature. 1976;261:333–5.
- Mazzeo RS, Grantham PA. Sympathetic response to exercise in various tissues with advancing age. J Appl Physiol. 1989;66:1506–8.
- Kawate R, Talan MI, Engel BT. Aged C57BL/6J mice respond to cold with increased sympathetic nervous activity in interscapular brown adipose tissue. J Gerontol. 1993;48:B180–3.
- McDonald RB, Hamilton JS, Horwitz BA. Influence of age and gender on brown adipose tissue norepinephrine turnover. Proc Soc Exp Biol Med. 1993;204:117–21.
- Scarpace PJ, Matheny M, Borst SE. Thermogenesis and mitochondrial GDP binding with age in response to the novel agonist CGP-12177A. Am J Physiol. 1992;262(2 Pt 1):E185–90.
- McDonald RB, Horwitz BA, Hamilton JS, Stern JS. Cold- and norepinephrine-induced thermogenesis in younger and older Fischer 344 rats. Am J Physiol. 1988;254(3 Pt 2):R457–62.
- Scarpace PJ, Mooradian AD, Morley JE. Age-associated decrease in beta-adrenergic receptors and adenylate cyclase activity in rat brown adipose tissue. J Gerontol. 1988;43:B65–70.
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–8.
- Cypess AM, Chen YC, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–5.
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013;110:12480–5.
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–52.
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123: 3395–403.
- Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J Nucl Med. 2013; 54:523–31.
- Boren J, Taskinen MR, Olofsson SO, Levin M. Ectopic lipid storage and insulin resistance: a harmful relationship. J Intern Med. 2013; 274:25–40.
- Guerra C, Navarro P, Valverde AM, Arribas M, Bruning J, Kozak LP, et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J Clin Invest. 2001; 108:1205–13.
- Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105.
- Bartelt A, Merkel M, Heeren J. A new, powerful player in lipoprotein metabolism: brown adipose tissue. J Mol Med (Berl). 2012;90: 887–93.
- Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol. 2013;167:2264–70.
- Rogers NH, Smith RG. Brown-to-white transition in subcutaneous fat: linking aging and disease. Aging. 2012;4:728–9.
- Timmons JA, Pedersen BK. The importance of brown adipose tissue. N Engl J Med. 2009;361:415–16; author reply 418–21.
- Hu HH, Gilsanz V. Developments in the imaging of brown adipose tissue and its associations with muscle, puberty, and health in children. Front Endocrinol (Lausanne). 2011;2:33.
