Abstract
Significant effects on blood pressure (BP) have been reported from large nutritional interventions, particularly the Dietary Approaches to Stop Hypertension (DASH) and the Mediterranean diet. In more recent years, numerous studies have investigated the possible BP-lowering effect of different nutraceuticals; these range from specific foods to minerals, lipids, whole proteins, peptides, amino acids, probiotics, and vitamins. While a very large body of evidence supports the use of potassium, L-arginine, vitamins C and D, cocoa flavonoids, beetroot juice, some probiotics, coenzyme Q10, controlled-release melatonin, aged garlic extract, and coffee, the use of other nutraceuticals, such as green tea, flaxseed, and resveratrol, has not as yet been supported by adequate evidence. In some cases, e.g. proteins/peptides, the responsible component needs also to be fully uncovered. Finally, while for most of the products only short-term studies are available, with no specific end-points, an ongoing very large prospective study on chocolate flavanols will answer the question whether this may reduce cardiovascular risk. Thus, in addition to data on long-term safety, further clinical research is advisable in order to identify, among active nutraceuticals, those with the best cost-effectiveness and risk–benefit ratio for a wide use in the general population with a raised cardiovascular risk consequent to uncomplicated hypertension.
A large number of nutraceuticals have shown blood pressure-lowering activity.
They range from commonly used dietary components, such as chocolate and beetroot juice, to vitamins, minerals, probiotics, coenzyme Q10, melatonin, dried garlic, and several others.
The long-term intake of these products, associated with an improved life-style, may reduce the number of subjects assigned to pharmacological treatment.
Introduction
Despite the availability of many effective and well-tolerated pharmacological treatments, cardiovascular (CV) diseases remain the leading cause of death and disability in developed countries (Citation1). Arterial hypertension is one of the most relevant independent risk factors for CV diseases, and incidence is expected to grow all over the world in the next two decades (Citation2). In particular, Framingham Study data indicate that the lifetime risk of developing hypertension is a staggering 90%, and it is estimated that the global burden of hypertension will increase to 1.56 billion affected individuals by 2025 (Citation2).
Elevated BP accounts annually for 7.6 million premature deaths and a loss of 92 million disability-adjusted life-years (1 disability-adjusted life-year is equivalent to 1 lost year of a healthy life) (Citation3). Further, numerous studies have shown that the maintenance of normal blood pressure (BP) levels reduces the incidence of CV complications, both in the hypertensive population and in patients whose BP values are only slightly elevated (Citation4). This underlies the importance of improving BP control in the general population. However, since it is not reasonable to treat all subjects with suboptimal BP control with antihypertensive medications, the major international guidelines have stressed the preventive impact of dietary and life-style intervention in order to reach and maintain optimal BP levels (Citation5,Citation6).
Large studies have shown the benefit of significant dietary changes on BP levels, among these the Dietary Approaches to Stop Hypertension (DASH) (Citation7) and the Mediterranean Diet studies (Citation8). A large number of studies have investigated also the BP-lowering activity of different dietary supplements, now generally labeled ‘nutraceuticals’. By definition, the term ‘nutraceutical’ indicates foods or parts of foods providing some medical or health benefits. The aim of this review is to provide available evidence supporting the use of some dietary supplements with known BP-lowering activity in the clinical practice.
Dietary components associated with lower blood pressure: proteins, lipids, minerals, vitamins
Proteins and peptides
The effects of dietary proteins on BP are attributable to complex mechanisms, being linked to the protein itself, to single peptides, or single amino acids. The intake of plant proteins (in particular soy protein) seems to be associated with mildly but significantly lower BP levels (Citation9), but it may not be easy to discriminate between the effects of the protein itself and of other protein-associated components. In particular, isoflavones associated with soy could be partly responsible: a recent meta-analysis showed that in hypertensive patients soy isoflavone intake is associated with a systolic BP (SBP) change by –5.94 mmHg (95% confidence intervals [CI] −10.55, −1.34; P = 0.01) and of diastolic BP (DBP) by –3.35 mmHg (95% CI −6.52, −0.19; P = 0.04) (Citation10).
However, the interest in peptides derived from food proteins is growing, due to the presence of angiotensin-converting enzyme (ACE) inhibitory peptides (Citation11), encrypted into the food protein sequences and possibly released by enzymatic hydrolysis and/or fermentation with suitable micro-organisms (Citation12). Active peptides must escape complete hydrolysis during digestion, a feature favored by the presence of one or more proline residues, especially when they are vicinal, as in the milk tripeptides Val-Pro-Pro and Ile-Pro-Pro (Citation13). There are, however, also technological solutions, e.g. micro- or nano-encapsulations, that may be useful in controlling degradation and release of peptide inhibitors of the ACE activity (Citation14).
While milk proteins, either casein or whey proteins, are good sources of BP-lowering peptides (Citation11), their role in BP control is as yet unclear. Milk proteins may be fermented by micro-organisms and proteolytic enzymes (alone or in combination) thus transforming proteins into bioactive peptides, commercialized not as pure compounds, but contained at a certain concentration in complex foods, such as yogurts or other fermented products. The stability of the chemical structure and the actual biological activity inside the food matrices are certainly relevant issues in this area that may justify the inconsistent results of experimental and clinical studies (Citation11). The best-known structures, i.e. the tripeptides Val-Pro-Pro and Ile-Pro-Pro, are available in two major commercial products, based on sour milk, i.e. the Finnish Evolus® and the Japanese Calpis®. These products have not achieved wide distribution outside of these countries, and there is still skepticism regarding their possible therapeutic use. Evaluation in three meta-analyses (Citation15–17) provided, further, somewhat inconsistent results. The oldest considered 14 randomized clinical trials: 13 on different milk protein derivatives and 1 on a bonito-fish hydrolyzed preparation (Citation15). The pooled effect of the peptide preparations was −5.13 mmHg (95% CI −7.12, −3.14) for SBP and −2.42 mmHg (95% CI −3.82, −1.03) for DBP. Interestingly, the fish preparation gave outcomes perfectly comparable to those of the milk preparations. The second meta-analysis considered 18 randomized clinical trials, all performed with milk products (Citation16) at daily doses corresponding to 5–60 mg of the tripeptides Val-Pro-Pro and Ile-Pro-Pro: the pooled effect of the peptides was a reduction of −3.73 mmHg (95% CI −6.70, −1.76) for SBP and 1.97 mmHg (95% CI −3.85, −0.64) for DBP. Interestingly, a separate analysis of the Asian and European studies indicated that the efficacy is much larger in Asian than in European subjects (Citation17). This stimulated a third meta-analysis dedicated specifically to European studies, again focused on the same milk tripeptides: the pooled change in SBP was −1.28 mmHg (95% CI −2.09,−0.48; P = 0.0017) and that in DBP was −0.59 mmHg (95% CI −1.18,−0.01; P = 0.047). In this study, a significant effect was observed for age, each additional year reducing the effect on SBP by 0.09 mmHg. Available data thus do not indicate that the inclusion of milk ACE-inhibitory peptides in the diet of moderately hypertensive subjects would provide significant benefit.
More recently, also peptides from plant proteins have been studied in detail with positive results (Citation18–20). Soybean (Citation19,Citation21), canola-seed (Citation22), pea (Citation23), peanut (Citation24), and hempseed protein (Citation25) are only a few examples of plant proteins currently under intense investigation. The activity depends not only on the starting material, but also on the enzyme used for hydrolysis. An example is presented in that shows the results of a study performed on the proteins of three lupin species (Citation26). Lupin is a leguminous plant with seeds containing up to 40% of arginine-rich proteins. After extraction, proteins were hydrolyzed with different enzymes, such as pepsin, trypsin, chymotrypsin, corolase PP®, umamizyme and flavourzyme, giving peptide mixtures, tested for their ACE-inhibitory activity. Peptic peptides were found to be the most effective.
Table I. ACE-inhibitory activities of lupin proteins hydrolyzed with different enzymes. IC50 values are reported in μg/mL as mean value ± standard deviation of three independent experiments (adapted from Boschin et al., 2014).
As for single amino acids, the most important case is that of L-arginine, a semi-essential amino acid, natural substrate for nitric oxide (NO) synthase, and responsible for the production of the endothelium-derived relaxing factor NO (Citation27). A recent meta-analysis of 11 randomized, controlled trials involving 387 participants with oral L-arginine doses from 4 to 24 g/day concluded that, compared to placebo, the intervention significantly lowered SBP by 5.39 mmHg (95% CI − 8.54, − 2.25; P = 0.001) and DBP by 2.66 mmHg (95% CI − 3.77, − 1.54; P < 0.001), a 4-week treatment being adequate to achieve a maximal effect.
Dietary lipids: omega-3 fatty acids and others
Dietary fats generally exert major roles in regulating plasma lipids, but have little effect on BP. However, a number of clinical trials evaluated different dosages of omega-3 polyunsaturated fatty acids (PUFAs), generally between 2 and 4 g/day. These, given as triglycerides or as ethyl esters, have been associated with a variable improvement of both SBP and DBP, generally around 4/2 mmHg (Citation28). A recent meta-analysis further showed that omega-3 intake is associated both with improved pulse wave velocity (standard mean difference = 0.33; 95% CI 0.12, 0.56; P < 0.01) and arterial compliance (standard mean difference = 0.48; 95% CI 0.24, 0.72; P < 0.001). No safety concerns were raised aside from mild gastrointestinal discomfort after high dosages (Citation29). A number of mechanisms have been suggested, ranging from enhanced NO generation, raised synthesis of vasodilator prostaglandins, reduced insulin-resistance, parasympathetic stimulation, and suppression of the renin–angiotensin–aldosterone system (Citation30).
Minerals for BP management
Major minerals positively involved in BP regulation are potassium, magnesium, and calcium. A balanced diet should contain 4,700 mg/day (120 mmol/day) potassium (K+) with a K+/Na+ ratio of about 4–5 to 1. Doubling the K+ intake is associated with a 4–8 mmHg SBP and 2.5–4 mmHg DBP reduction in hypertensive subjects. The response seems to be higher in blacks and in patients with a higher dietary Na+ intake (Citation31). A higher K+ is also associated with a lower incidence of CV and cerebrovascular accidents, type 2 diabetes, left ventricular hypertrophy, heart failure, and cardiac arrhythmias, independent of BP reduction (Citation32).
A recent meta-analysis concluded that a 1.64 g (42 mmol) per day higher K+ intake is associated with a 21% lower risk of stroke (RR 0.79; 95% CI 0.68, 0.90; P = 0.0007), with a trend toward a lower risk of coronary and total CV disease (RR 0.93; 95% CI 0.87, 0.99; P = 0.03 and RR 0.74; 95% CI 0.60, 0.91; P = 0.0037) (Citation33). It has been estimated that each 1 g increase in daily K+ intake reduces all-cause mortality by 20% and each 1 g decrease in daily Na+ intake decreases all-cause mortality by 20% (Citation34).
The K+ induced BP reduction may be related to increased natriuresis, modulation of baroreflex sensitivity, decreased sensitivity to catecholamines and angiotensin II, increased sodium-potassium ATPase in the vascular smooth muscle cells, and decreased NADPH oxidase. This last-mentioned will lower oxidative stress and inflammation, improve insulin sensitivity, decrease asymmetric dimethylarginine, reduce intracellular Na+, and reduce production of tumor growth factor-β (Citation35).
There is also an inverse relationship between dietary magnesium (Mg++) intake and BP. Clinical trials tested a dose range of 120–973 mg (mean dose 410 mg) with a follow-up of 3 to 24 weeks. The maximum BP reduction was 5.6 ± 2.2 / 2.8 ± 1.9 mmHg, with a large variability. However, a recent meta-analysis of randomized clinical trials concluded that Mg++ supplementation is associated with a decrease in SBP of 3–4 ± 2 mmHg and DBP of 2.5 ± 1 mmHg; better responses were reported in cross-over trials and with intakes > 370 mg/day (Citation36). The combination of high K+ and low Na+ with increased Mg++ shows additive anti-hypertensive effects in both drug-treated and untreated hypertensives (Citation37).
The Mg++-induced BP reduction may be linked to calcium-channel blockade, increased PGE1, and NO synthesis. The optimal dose seems to be between 500 and 1,000 mg per day, possibly chelated to an amino acid to improve absorption and decrease the incidence of diarrhea. Adding e.g. taurine at 1 to 2 g per day seems to enhance the anti-hypertensive effects of Mg++ (Citation38).
While in hypertensives Ca++ supplementation seems not to be effective, it may be useful in pregnant women: a recent meta-analysis of the Cochrane Collaboration, involving 13 studies and more than 15,700 women, supports the use of Ca++ (500–600 mg/day) in pregnancy, since it appears to reduce the risk of pre-eclampsia to almost one half, as well as the risk of preterm birth (Citation39).
Vitamins with BP-regulating activity
Among vitamins, plasma ascorbate concentrations in humans are inversely related to BP (Citation40). In particular, hypertensive subjects have significantly lower ascorbate compared to normotensives (40 μmol/L versus 57 μmol/L, respectively) (Citation41). A depletion–repletion study of vitamin C also confirmed an inverse correlation of plasma ascorbate with SBP and DBP (Citation42). Thus, in order to achieve a positive effect on BP, an ascorbate serum level of at least 100 μmol/L is recommended (Citation43). A recent meta-analysis of clinical trials with a median daily vitamin C dose of 500 mg over an 8-week period reported a reduction of SBP by 4.85 ± 1.21 mmHg (P < 0.01) and of DBP by 1.67 ± 0.72 mmHg (P = 0.17) in hypertensives; no safety concerns were raised (Citation44). Vitamin C also seems to improve the efficacy of some antihypertensive drugs, such as amlodipine (Citation45).
Numerous mechanisms have been hypothesized for the vitamin C-induced BP reduction: raised NO and PGI2 with improved endothelial function and arterial compliance, induction of diuresis, reduced adrenal steroid production, improvement of the sympathovagal balance, raised Na/K ATPase, superoxide dismutase, and cyclic GMP, activation of K+ channels, and reduction of cytosolic calcium (Citation46) and of serum aldehydes (Citation47). Moreover, vitamin C may decrease the binding affinity of the angiotensin 1 receptor (ATR1) for angiotensin II by disrupting the ATR1 disulfide bridges (Citation48). The supplemental doses of vitamin C suggested to improve BP (500–1,000 mg/day) are usually well tolerated.
Patients with hypertension are also more likely than controls to have reduced levels of vitamin D (Citation49). However, beyond some suggestive experimental and observational studies, a relatively large number of recent meta-analyses of the available clinical data have provided only conflicting data on the effects of vitamin D on BP (Citation50).
Use of probiotics for blood pressure control
Interest on the health benefits of probiotics has grown in the past several years. Probiotics are defined as live micro-organisms that may exert health benefits for the recipients, if consumed in adequate amounts. They have been studied particularly for improving immune system functions and preventing diarrhea (Citation51). Probiotics can also improve BP by mechanisms involving total and low-density lipoprotein cholesterol (LDL-C) reduction, reduced glucose and insulin resistance, and regulation of the ACE system (Citation52,Citation53).
In order to assess the comparative benefit of probiotics based on species, duration, or dose of consumption, more recently Khalesi et al. (Citation54) evaluated in an updated meta-analysis nine randomized controlled trials. Four of these used yogurt as the source of probiotic bacteria, two used fermented and sour milk, one capsulated probiotic supplements, one probiotic rose-hip drinks, and another probiotic cheese. Four studies used a single species of probiotics, whereas the others used a combination of two or three strains. Total daily dose varied from 109 colony-forming units (CFU) to 1012 CFU, and durations were from 3 to 9 weeks. There were no major side effects, and compliance was adequate. The result was that SBP was changed by −3.56 mmHg (95% CI −6.46, −0.66) and DBP by −2.38 mmHg (95% CI −3.84, −0.93). Multiple species of probiotics appeared to exert a higher efficacy, and a probiotic treatment was more effective in patients with baseline BP ≥ 130/85 mmHg, particularly with consumption of probiotic doses ≥ 1011 CFU, duration of intervention ≥ 8 weeks, and multiple species of probiotics (Citation54).
Foods with specific BP-lowering activity
Cocoa/chocolate
The understanding of cocoa/chocolate as effective nutraceutical tools for the management of hypertension started from a clinical study on the Kuna Indians, living in an indigenous island setting (Ailigandi) off the coast of Panama, where documentation of freedom of hypertension as well as of CV diseases was reported as early as in the 1940s (Citation55). This appeared not to be genetic but rather influenced by environmental exposure. High BP has been, in fact, documented among migrant Kunas (Citation56). The dietary intake of Kunas, stable over time, was evaluated comparing Ailigandi and Vera Cruz, a suburb of Panama City. It came out that Ailigandi Kunas consume a 10-fold higher amount of cocoa-containing beverages, 4-fold more of fish, and twice the amount of fruit as urban Kunas (all P < 0.001) (Citation57), with no difference in salt and urinary sodium.
Clinical and experimental studies on the mechanism of the hypotensive activity of cocoa/chocolate
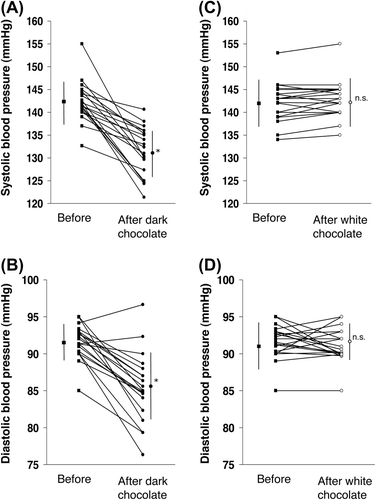
A large number of investigations have tested the mechanisms underlying the reduced BP and increased flow-mediated dilatation (FMD) following chocolate. It is now clear that, whereas a hypotensive effect is exerted by the dark chocolate (DC) (i.e. a flavanol-rich product), this may not be the case for white chocolate (WC) (Citation58). Flavanols from DC provide a powerful antioxidant activity by releasing (-)epicatechin into the bloodstream, (Citation59), but later studies underlined that a significant BP reduction occurs after the flavanol-rich DC, not after WC, concomitant with improvement of FMD () (Citation60) and a modest reduction of serum LDL-cholesterol after DC (Citation61).
Figure 1. Effects of dark chocolate (100 g/day) versus white chocolate (90 g/day) given in cross-over design for 15 days each to 20 never-treated essential hypertensives. In all panels individual data are given, as well as vertical lines indicating means ± SD. Asterisks indicate significant differences between dark chocolate versus white chocolate and baseline values (P < 0.05) (Citation60).
A significant increase in S-nitrosoglutathione, a product of raised unstable NO reacting with thiol groups, was reported after DC intake (Citation62) associated both with an improvement in coronary vasomotion (10%–15% increase in diameter) as well as in platelet adhesion (Citation63). Interestingly, just 90 min after a cocoa drink with 821 mg flavanols/day given for 5 days, a remarkable increase of pulse amplitude is noted, a marker of vasodilation consequent to activation of the NO system, antagonized by prior administration of L-NAME (Citation64). Schroeter et al. (Citation65) reported that the dramatic elevation of circulating NO species and the enhanced FMD response are directly linked to a rise of (-)epicatechin and of its metabolite epicatechin-7-O-glucuronide, both independent predictors of the vascular effects. A mixture of flavanol metabolites resembling the profile and concentration of circulating flavanols after DC ingestion induced the relaxation of preconstricted rabbit arteries ex vivo (Citation65). A number of studies have tested DCs with different polyphenol contents. A higher polyphenol content is associated with improved reduction of BP as well as of other markers of metabolic abnormality, e.g. LDL-cholesterol (Citation66), insulin sensitivity (Citation61), and C-reactive protein concentrations (Citation67). Even a low habitual cocoa intake (just 6.3 g/day of DC containing 30 mg of polyphenols) for 18 weeks can reduce BP and increase S-nitrosoglutathione concentrations (Citation62). A CV benefit may well be consequent to an improved coronary circulation, as evaluated by transthoracic Doppler echocardiography in healthy adults following a flavanol-rich DC (Citation68).
Epidemiological studies and associated benefits
The epidemiological studies have been generally based on populations with a low chocolate intake; still, findings appear to be consistent with the hypothesis of CV benefit. Reduced mortality after myocardial infarction (MI) was reportedly associated with chocolate intake in the Stockholm Heart Epidemiology Program (Citation69). In a follow-up of 1,169 non-diabetics with a confirmed acute MI between 1991 and 1994, a self-reported usual chocolate consumption over the preceding 12 months was associated with hazard ratios between 0.73 (chocolate less than once per month) to 0.34 (twice or more per week) in the following 8 years.
The reduction of CV risk has been confirmed in a study on German adults. Chocolate consumption assessed in 19,457 adults followed for about 8 years lowered the relative risk of the combined outcome of MI and stroke from top versus bottom quartiles by 0.61, the baseline BP explaining 12% of this lower risk (Citation70). Milk chocolate was the most frequently consumed, followed by DC.
In view of the reported links between raised BP and impaired cognitive function in the elderly (Citation71), the Cocoa, Cognition and Aging Study (Citation72), in addition to BP reduction, reported a significant improvement in verbal fluency tests after DC, not after WC. In a follow-up study, the authors also noted a significant improvement in insulin sensitivity after DC, explaining part of the cognitive improvement (Citation73).
Chocolate consumption appears therefore to be a potentially effective tool to improve cardiometabolic disorders. A systematic review (Citation74) reported that the highest levels of chocolate consumption are associated with a 37% reduction in CV disease and a 9% reduction in stroke risk compared with the lowest level of intake. Zomer et al. (Citation75) also evaluated the effectiveness of DC consumption as a preventive treatment for people at a high risk of CV disease, concluding that 85 CV events per 10,000 population can be reduced by polyphenols equivalent to 100 g per day of DC over 10 years.
Recently, however, the protective role of polyphenols has been the object of criticism. A comparison of a 4-week intake of a high-flavanol chocolate versus a normal flavanol chocolate showed that both improved FMD (by 1%), paralleled by a decreased arterial augmentation index, a validated index of arterial rigidity. This study, carried out in overweight men, thus suggests that flavanol enrichment may not be crucial in the CV advantage of DC and may possibly lead to a more bitter chocolate, negatively affecting consumption (Citation76). Whether this is the real case is hard to conclude.
Regulation of chocolate: medical indications and future directions
All the reported findings have led to a very positive view on DC from health authorities. The health benefits of cocoa/chocolate were, in fact, the object of two separate Scientific Opinions by the European Food Safety Authority (EFSA). EFSA provides mandatory opinions on health claims related to different foods. Claims are otherwise prohibited in Europe.
The first Scientific Opinion, given in 2012 (Citation77) concluded that chocolate flavanols are sufficiently characterized and endothelial dependent vasodilation is a beneficial physiological effect. The Committee suggested the following wording: ‘Cocoa flavanols help maintain endothelium-dependent vasodilatation, which contributes to normal blood flow’. The EFSA panel indicated that, in order to obtain the claimed effect, 200 mg of cocoa flavanols should be consumed daily and this could be provided by 2.5 g of high flavanol cocoa powder or 10 g of high-flavanol (HF) DC.
More recently, a second Scientific Opinion (Citation78) extended the use of the Health Claim to HF cocoa extracts to be consumed in capsules, tablets or in other foods including beverages. The 200 mg of cocoa flavanols consumed daily could be provided by less than 1 g of HF cocoa extract with an appropriate formulation and within a balanced diet.
The final evidence on the benefit of chocolate polyphenols in improving CV health and preventing CV disease will be provided by the ongoing prevention study, coordinated by the Department of Epidemiology of the Brigham and Women University, Boston, USA, and supported by MARS Symbioscience. The COSMOS (COcoa Supplement and Multivitamin Outcomes Study) will investigate 12,000 women aged ≥ 65 years and 6,000 men aged ≥ 60 years randomized either to placebo capsules or to the cocoa flavanols (600 mg/day). This clinical trial will have 4 years of follow-up and will evaluate the effects of flavanols in reducing the risk of major CV events, as well as of cancer, bone disease, diabetes, heart failure, sarcopenia, and age-related macular degeneration.
Beetroots
Health benefits from a diet rich in fruits and vegetables are likely to be multifactorial, but specific food groups, such as green leafy vegetables, may provide the greatest protection against CVD and ischemic stroke (Citation79). Among these, beetroot (Beta vulgaris) is possibly the highest nitrate-accumulating vegetable. Increasing evidence suggests that NO can be biosynthesized in vivo following intake of inorganic nitrate: a study, in healthy volunteers, showed reduced DBP after sodium nitrate (0.1 mmol kg/body weight per day) and suggested beetroot juice as a possible source (Citation80). Nitrate will follow a process independent of that generally followed by the body, i.e. conversion of L-arginine to NO by the NO synthase pathway. Ingested nitrate is instead converted to nitrite by commensal Gram negative bacteria in the oral cavity and absorbed in the stomach (Citation81); it is finally reduced to NO in the vessel wall and erythrocytes, and this leads to vasodilatation.
Beetroot is also rich in betalains, nitrogen-containing color compounds not commonly present in edible plants. Betalains are generally divided into red/purple beta-cyanins, responsible for the color of red beetroot, and yellow/orange beta-xanthins that contribute to the color of yellow beetroot. Betalains may act as antioxidants by the donation of electrons, possibly suggesting a role in protection against oxidative stress-related diseases, hypertension, and CV diseases (Citation82).
A number of reports have provided evidence of a BP-reducing activity of beetroot products in normotensives and hypertensives. Hobbs et al. (Citation83), in a single-blind cross-over postprandial study in normotensives with either beetroot juice (100, 250, 500 g) or bread products with red and white beetroot, reported a rapid and marked reduction in both SBP and DBP, maximal with 250 and 500 g beetroot juice (lowering of −20.5/–22.2 mmHg). Similarly, beetroot-enriched bread resulted in a significant reduction of SBP and DBP in a similar range. The presence or absence of betalains did not appear to have any impact on the BP-lowering activity.
More recently and more importantly, Ghosh et al. (Citation84) reported an activity of beetroot juice (BJ) in drug-naïve hypertensive individuals. The mechanism was also evaluated in a parallel study in spontaneously hypertensive rats: in these, the hypotensive activity was abolished by inhibiting xanthine oxidoreductase (XOR), dependent on the nitrite-reductase activity in erythrocytes. Hypertensives used a commercial BJ-enriched drink, containing approximately 3.5 mmol nitrate in 250 mL, i.e. just below the threshold dose of nitrate (4 mmol) in previous studies (Citation80). After BJ administration, a highly significant mean reduction of both SBP and DBP in individuals with a baseline SBP around 140 mmHg was detected. The BP reduction was well maintained for all the 24 h of monitoring (), associated with a reduced pulse velocity and increased erythrocyte XOR expression. These very positive findings have resulted in a wide distribution of this BJ formulation, particularly in the UK.
Figure 2. Effects of beetroot juice (250 mL) in grade 1 untreated hypertensive patients (n = 15, mean off-treatment BP 139.9/86.5 mmHg). Means ± SEM of ΔSBP, ΔHR, and Δnitrate are given (Citation84).
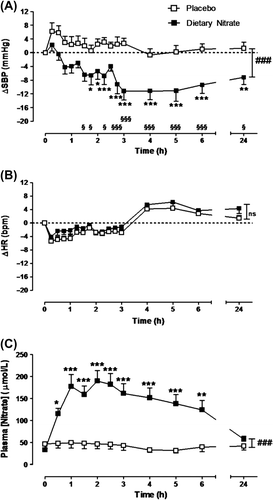
Very recently, the same preparation (250 mL daily as BJ) was tested in a double-blind placebo-controlled study lasting 4 weeks in hypertensives. The mean clinic BP was reduced by 7.7 ± 2.4 mmHg and the 24 h ambulatory BP by 7.7 ± 5.2 mmHg (Citation85). A better efficacy was found in home monitoring with no evidence of tachyphylaxis over the 4-week period. Endothelial function improved by 20%, and arterial stiffness was reduced by 0.59 m/s. The durable BP reduction with BJ confirms this as an affordable, readily available adjunctive treatment for hypertension.
Interestingly, the use of BJ has been recently associated with an improved muscle blood flow during muscular contractions, thus resulting in potentially improved performance. A single dose (70 mL containing approximately 5 mmol nitrate) proved to enhance cycling performance in trained cyclists exposed to ‘simulated altitude’ (Citation86). Altitude may result in a reduction of exhaled NO (suggesting reduced NO production). Kelly et al. (Citation87) confirmed these findings, by showing that the rate of decline of plasma NO2- is greater during severe intensity exercise and nitrate supplementation enhances VO2 kinetics. More recently, however, the beneficial effects of BJ could not be confirmed in a longer trial in well trained runners in normobaric hypoxia (Citation88), thus pointing to the potential limits of this nutraceutical in performance improvement.
Other nutraceuticals with well-established BP-lowering activity
Coffee intake has been frequently associated with reduced BP (Citation89), although epidemiological data have not consistently shown a beneficial effect. Some clinical trials have shown that chlorogenic acid from non-roasted green coffee is associated with a significant BP reduction (Citation90). Roasting of ordinary coffee induces the synthesis of hydroxyhydroquinone (HHQ) that inhibits the effect of chlorogenic acid, so that coffee may reduce BP inversely to the HHQ content (Citation91).
The only controlled investigation, on normotensive individuals, evaluated green coffee versus black coffee (Citation92). Green coffee (GC) significantly reduced SBP (−2.65 ± 1.37 mmHg) versus a non-significant reduction with black coffee (BC). DBP was instead significantly reduced by both (respectively −3.1 ± 0.81 for GC and −2.9 ± 1.05 mmHg for BC), and both reduced arterial elasticity. These changes were associated with a significant reduction of urinary free cortisol. It is expected that better results may be obtained in hypertensives, not yet investigated.
Olive oil consumption, a cornerstone of the Mediterranean diet, is associated with a BP-reducing effect, not related to the dietary fatty acid (oleic acid has no BP-reducing activity) but to the polyphenol content (Citation93). Recently considerable interest has been raised on ethanol extracts from olive leaves that can be formulated into tablets. Olive leaves contain, in fact, hydroxytyrosol, oleanoic acid, and related triterpenoids, responsible, among others, for antihypertensive effects. In an open study on 40 borderline hypertensive monozygotic twins assigned to different groups receiving 500 or 1,000 mg/day olive leaf extracts for 8 weeks, BP changed significantly depending on dose, with mean SBP differences of ≤ 6 mmHg (500 mg) and ≤ 13 mmHg (1,000 mg), and DBP differences of ≤ 5 mmHg (Citation94). In a further randomized clinical trial, olive leaf extract, at the dosage regimen of 500 mg b.i.d., was similarly effective in lowering SBP and DBP in subjects with stage-1 hypertension as captopril, at the effective dose of 12.5–25 mg b.i.d. (Citation95).
The carotenoid lycopene has been the object of a number of reports and of randomized clinical trials. A recent meta-analysis of randomized clinical trials investigating the effect of lycopene (10–20 mg/day) on systolic BP suggested a significant BP-reducing effect (mean systolic SBP −5.60 ± 5.26 mmHg; P = 0.04) (Citation96). The effect of lycopene on BP appears to be additive to that of antihypertensive drugs (Citation97). Interestingly, availability of a lycopene-containing formulation of dark chocolate, apparently enhancing the availability of cocoa flavanols, improves the BP reduction (mean of −6.22 mmHg; 95% CI 5.00, 8.00) compared with regular DC (−3.0 mmHg; P < 0.05) (Citation98).
Aged dry garlic extract contains components (mainly allicin, flavonoids, and sulfur compounds) with ACE-inhibitory and calcium channel-blocking activity. These reduce catecholamine sensitivity, increase bradykinin and NO, and improve arterial compliance (Citation99). A recent meta-analysis of randomized, placebo-controlled trials shows a mean decrease of 4.6 ± 2.8 mmHg for SBP in the garlic group compared to placebo (P = 0.001) in normotensives, versus mean reductions of 8.4 ± 2.8 mmHg for SBP (P < 0.001), and 7.3 ± 1.5 mmHg for DBP (P < 0.001) in hypertensives. Regression analysis revealed a significant association between BP at the start of the intervention and BP reduction (Citation99). The effect seems to be additive to that of antihypertensive drugs (Citation100).
Other nutraceuticals not strictly taken with food but potentially active on BP levels are coenzyme Q10, pycnogenol, and melatonin. Coenzyme Q10 (ubiquinone) is a potent lipid phase antioxidant and free-radical scavenger. It regenerates other vitamins and antioxidants, reduces LDL oxidation, and is a co-factor in mitochondrial oxidative phosphorylation (Citation101). A meta-analysis of randomized, placebo-controlled, clinical trials concluded that oral treatment with 100 mg or more in subjects with SBP > 140 mmHg or DBP > 90 mmHg resulted in mean decreases in SBP of 11 mmHg (95% CI 8, 14) and DBP of 7 mmHg (95% CI 5, 8), usually after 4 weeks of treatment (Citation102). The main problem with coenzyme Q10 (CoQ10) is the cost of the high doses needed to obtain a significant BP reduction, because of the low bioavailability of CoQ10 in humans. This could be improved by the use of partial CoQ10 emulsification or the use of nanoparticles (Citation103).
The bark extract of Pinus pinaster (French maritime pine), usually marketed as pycnogenol, acts as a natural ACE-inhibitor, protects cell membranes from oxidative stress, increases NO, improves endothelial function and renal cortical blood flow, and decreases hs-CRP, all properties supporting a potential positive effect on BP (Citation104). Supplementation with 100 mg pycnogenol in subjects treated with different antihypertensive agents leads to a reduction of drug doses in nearly half of patients (Citation105,Citation106). A relatively old study indicated that pycnogenol 200 mg/day treatment in mildly hypertensive subjects can lead to a significant reduction of SBP but not of DBP under randomized placebo-controlled conditions (Citation107). More recently, the interest in pycnogenol has been directed to improved arterial function in heart failure patients, not associated, however, with BP reduction (Citation108).
Melatonin is the hormone normally secreted from the pineal gland at night and plays a pivotal role in the physiological regulation of circadian rhythms, including sleep. Melatonin appears to improve BP control by central and peripheral mechanisms, protecting vessels from oxidation, improving NO metabolism and consequently endothelial function (Citation109). A recent meta-analysis of randomized, double-blind, placebo-controlled trials (three with controlled-release and four with rapid-release melatonin) on 221 participants reported that nocturnal SBP decreases significantly with controlled-release melatonin (−6.1 mmHg; 95% CI −10.7, −1.5; P = 0.009), but not fast-release melatonin (−0.3 mmHg; 95% CI −5.9, 5.30; P = 0.92). Similar changes were noted for DBP. No safety concerns were raised (Citation110). Since beta-blockers inhibit melatonin secretion, supplementation may improve sleep in hypertensives treated with beta-blockers (Citation111).
Nutraceuticals with inadequately supported BP-lowering activity
A number of nutraceuticals have been reported to lower BP, mainly in non-controlled investigations or on a limited number of patients. While for some, e.g. flaxseed, a nutrigenetic related variability is probably in play, for others the BP-reducing activity may be too modest to be clinically significant.
α-Linolenic acid (ALA)-rich food items, such as flaxseed (linseed), have been evaluated with not well-defined results. Flaxseed provides 41% fat, 57% of which is α-ALA; with a smaller amount of other omega-3, lignans, and fiber, flaxseed has a number of possible health benefits, including BP reduction (Citation112). A recent meta-analysis of 14 randomized clinical trials indicates that flaxseed supplementation modestly reduces SBP (−1.77 mmHg; 95% CI −3.45, −0.09 mmHg; P = 0.04) and DBP (−1.58 mmHg; 95% CI −2.64, −0.52 mmHg; P = 0.003), independent of baseline BP. DBP seems to be particularly sensitive to whole flaxseed (−1.93 mmHg; 95% CI −3.65, −0.21 mmHg; P < 0.05), with improved efficacy with duration of consumption ≥ 12 weeks (−2.17 mmHg; 95% CI −3.44, −0.89 mmHg; P < 0.05) (Citation113). Flaxseed is thus not a nutraceutical with well-defined BP-lowering properties. Differently from standard omega-3, flaxseed may in fact act by altering circulating oxylipins via inhibition of a soluble epoxide hydrolase (Citation114). This bioactivity is nutrigenetically regulated: patients exhibiting a decrease in total plasma soluble epoxide hydrolase-derived oxylipins show a marked reduction in SBP (−7.97 mmHg; 95% CI −14.4, −1.50 mmHg), whereas those with increased oxylipins exhibit instead raised SBP.
Resveratrol, a natural antioxidant, is particularly concentrated in some qualities of red wine, stimulates endothelial NO production, reduces oxidative stress, inhibits vascular inflammation, and prevents platelet aggregation (Citation115). Recently, a reduction in arterial wall stiffening in primates was described (Citation116) associated with reduced BP. Unfortunately, the clinical evidence of a modulating effect of resveratrol on BP is inconclusive, probably related to the low bioavailability in humans (Citation117). A meta-analysis of nine randomized, double-blind, placebo-controlled trials including 390 subjects showed that grapeseed extract moderately but significantly lowered SBP (weighted mean difference −1.54 mmHg; 95% CI −2.85, −0.22; P = 0.02), but not DBP (Citation118), thus not allowing to select resveratrol among the potentially effective BP lowering nutraceuticals. A very recent study (Citation119) confirmed that resveratrol supplementation exerted minimal effects on lipid and no significant effect on BP.
Green tea has powerful antioxidant and anti-inflammatory activities, known to preserve and improve the endothelial function. A recent meta-analysis of 20 randomized clinical trials, with 1,536 participants, revealed a moderate but significant reduction in SBP, favoring green tea over placebo (−1.94 mmHg; 95% CI −2.95, −0.93; P = 0.0002) (Citation120). A further meta-analysis has reported more significant SBP and DBP reductions with green tea consumption exceeding 12 weeks (Citation121).
An interesting very recent trial has shown that adding a mix of flavonoids from dark chocolate, dehydrated red apple, and green tea (425.8 ± 13.9 mg epicatechin equivalents) to an antihypertensive drug regimen based on telmisartan or captopril improved the BP-lowering effect of both. In particular, captopril-treated patients improved their BP by −7/–5 mmHg, and the telmisartan-treated ones by −4/–3 mmHg, with a parallel improvement of triglycerides and hs-CRP (Citation122). BP changes, although statistically significant, do not appear to be adequate for advising green tea in BP management.
Conclusions
A number of nutraceuticals appear to be useful in the management of pre-hypertensive or stage-1 hypertensive patients, in association with an improvement of diet and life-style. Beyond a large number of dietary supplements that have shown an antihypertensive activity in single trials (i.e. soluble fibers, vitamin B6, pomegranate juice, sesame, alpha-lipoic acid, hawthorn extracts, and others) (Citation123), a number of well-designed studies support the use of potassium, L-arginine, vitamin C, cocoa flavonoids, beetroot juice, coenzyme Q10, controlled-release melatonin, and aged garlic extract. There is, however, still a need for data on the long-term safety for most of these products. Further, clinical research is also needed to identify, among active nutraceuticals, those with the best cost-effectiveness and lowest risk–benefit ratio for a wider use in the general population with significant CV risk. However, the best choice may be possibly to include several of these products in the daily diet in rotation, to improve long-term compliance in the dietary treatment of hypertension.
Declaration of interest: The authors report no conflicts of interest.
References
- Gaziano TA. Cardiovascular disease in the developing world and its cost-effective management. Circulation. 2005;112:3547–53.
- Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23.
- Lawes CM, Vander Hoorn S, Rodgers A;International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–18.
- McInnes GT. Lowering blood pressure for cardiovascular risk reduction. J Hypertens Suppl. 2005;23:S3–8.
- Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Pressure. 2014;23:3–16.
- Appel LJ, Giles TD, Black HR, Izzo JL Jr, Materson BJ, Oparil S, et al. ASH position paper: dietary approaches to lower blood pressure. J Am Soc Hypertens. 2010;4:79–89.
- Miller ER 3rd, Erlinger TP, Appel LJ. The effects of macronutrients on blood pressure and lipids: an overview of the DASH and OmniHeart trials. Curr Atheroscler Rep. 2006;8:460–5.
- Domenech M, Roman P, Lapetra J, Garcia de la Corte FJ, Sala-Vila A, de la Torre R, et al. Mediterranean diet reduces 24-hour ambulatory blood pressure, blood glucose, and lipids: one-year randomized, clinical trial. Hypertension. 2014;64:69–76.
- Altorf-van der Kuil W, Engberink MF, Brink EJ, van Baak MA, Bakker SJ, Navis G, et al. Dietary protein and blood pressure: a systematic review. PLoS One. 2010;5:e12102.
- Liu XX, Li SH, Chen JZ, Sun K, Wang XJ, Wang XG, et al. Effect of soy isoflavones on blood pressure: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2012;22:463–70.
- Korhonen H. Milk-derived bioactive peptides: from science to applications. Journal of Functional Foods. 2009;1:177–87.
- Hernandez-Ledesma B, del Mar Contreras M, Recio I. Antihypertensive peptides: production, bioavailability and incorporation into foods. Adv Colloid Interface Sci. 2011;165:23–35.
- Vermeirssen V, Van Camp J, Verstraete W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br J Nutr. 2004;92:357–66.
- He R, Aluko RE, Ju XR. Evaluating molecular mechanism of hypotensive peptides interactions with renin and angiotensin converting enzyme. PLoS One. 2014;9:e91051.
- Pripp AH. Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials. Food Nutr Res. 2008;52.
- Cicero AF, Gerocarni B, Laghi L, Borghi C. Blood pressure lowering effect of lactotripeptides assumed as functional foods: a meta-analysis of current available clinical trials. J Hum Hypertens. 2011;25:425–36.
- Cicero AF, Aubin F, Azais-Braesco V, Borghi C. Do the lactotripeptides isoleucine-proline-proline and valine-proline-proline reduce systolic blood pressure in European subjects? A meta-analysis of randomized controlled trials. Am J Hypertens. 2013;26:442–9.
- Udenigwe CC, Aluko RE. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J Food Sci. 2012;77:R11–24.
- Boschin G, Scigliuolo GM, Resta D, Arnoldi A. ACE-inhibitory activity of enzymatic protein hydrolysates from lupin and other legumes. Food Chem. 2014;145:34–40.
- Arnoldi A, Zanoni C, Lammi C, Boschin G. The role of grain legumes in the prevention of hypercholesterolemia and hypertension. Critic Rev Plant Sci. 2015;34:144–68.
- Wu J Ding X. Characterization of inhibition and stability of soy- protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res Int. 2002;35:367–75.
- Wu J, Aluko RE, Muir AD. Production of angiotensin I-converting enzyme inhibitory peptides from defatted canola meal. Bioresour Technol. 2009;100:5283–7.
- Li H, Prairie N, Udenigwe CC, Adebiyi AP, Tappia PS, Aukema HM, et al. Blood pressure lowering effect of a pea protein hydrolysate in hypertensive rats and humans. J Agric Food Chem. 2011;59:9854–60.
- Quist EE, Phillips RD, Saalia FK. Angiotensin converting enzyme inhibitory activity of proteolytic digests of peanut (Arachis hypogaea L.) flour. LWT Food Science and Technology. 2009;42:694–9.
- Girgih AT, Udenigwe C, Li H, Adebiyi AP, Aluko RE. Kinetics of enzyme inhibition and antihypertensive effects of hemp seed (Cannabis sativa L.) protein hydrolysates. J Am Oil Chem Soc. 2011;88:1767–74.
- Boschin G, Scigliuolo GM, Resta D, Arnoldi A. Optimization of the enzymatic hydrolysis of lupin (Lupinus) proteins for producing ACE-inhibitory peptides. J Agric Food Chem. 2014;62:1846–51.
- Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36:249–55.
- Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol. 2009;7:330–7.
- Pase MP, Grima NA, Sarris J. Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br J Nutr. 2011;106:974–80.
- Balakumar P, Taneja G. Fish oil and vascular endothelial protection: bench to bedside. Free Radic Biol Med. 2012;53:271–9.
- Whelton PK, He J. Potassium in preventing and treating high blood pressure. Semin Nephrol. 1999;19:494–9.
- Gu D, He J, Wu X, Duan X, Whelton PK. Effect of potassium supplementation on blood pressure in Chinese: a randomized, placebo-controlled trial. J Hypertens. 2001;19:1325–31.
- D’Elia L, Barba G, Cappuccio FP, Strazzullo P. Potassium intake, stroke, and cardiovascular disease a meta-analysis of prospective studies. J Am Coll Cardiol. 2011;57:1210–19.
- Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183–91.
- Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13:309–17.
- Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr. 2012;66:411–18.
- Laurant P, Touyz RM. Physiological and pathophysiological role of magnesium in the cardiovascular system: implications in hypertension. J Hypertens. 2000;18:1177–91.
- Houston MC. The role of magnesium in hypertension and cardiovascular disease. J Clin Hypertens. 2011;13:843–7.
- Hofmeyr G, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2014;6:CD001059.
- Ness AR, Khaw KT, Bingham S, Day NE. Vitamin C status and blood pressure. J Hypertens. 1996;14:503–8.
- Ness AR, Chee D, Elliott P. Vitamin C and blood pressure—an overview. J Hum Hypertens. 1997;11:343–50.
- Block G, Mangels AR, Norkus EP, Patterson BH, Levander OA, Taylor PR. Ascorbic acid status and subsequent diastolic and systolic blood pressure. Hypertension. 2001;37:261–7.
- Sherman DL, Keaney JF Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936–41.
- Juraschek SP, Guallar E, Appel LJ, Miller ER 3rd. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:1079–88.
- Mahajan AS, Babbar R, Kansal N, Agarwal SK, Ray PC. Antihypertensive and antioxidant action of amlodipine and vitamin C in patients of essential hypertension. J Clin Biochem Nutr. 2007;40:141–7.
- Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–7.
- Hatzitolios A, Iliadis F, Katsiki N, Baltatzi M. Is the anti-hypertensive effect of dietary supplements via aldehydes reduction evidence based? A systematic review. Clin Exp Hypertens. 2008;30:628–39.
- Leclerc PC, Proulx CD, Arguin G, Belanger S, Gobeil F Jr, Escher E, et al. Ascorbic acid decreases the binding affinity of the AT1 receptor for angiotensin II. Am J Hypertens. 2008;21:67–71.
- Kim DH, Sabour S, Sagar UN, Adams S, Whellan DJ. Prevalence of hypovitaminosis D in cardiovascular diseases (from the National Health and Nutrition Examination Survey 2001 to 2004). Am J Cardiol. 2008;102:1540–4.
- Vaidya A, Forman JP. Vitamin D and vascular disease: the current and future status of vitamin D therapy in hypertension and kidney disease. Curr Hypertens Rep. 2012;14:111–19.
- McFarland LV. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol. 2006;101:812–22.
- Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, et al. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003;67:1421–4.
- Seppo L, Jauhiainen T, Poussa T, Korpela R. A fermented milk high in bioactive peptides has a blood pressure-lowering effect in hypertensive subjects. Am J Clin Nutr. 2003;77:326–30.
- Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903.
- Kean B. The blood pressure of the Kuna Indians. Am J Trop Med Hyg. 1944;24:341–3.
- Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, et al. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension. 1997;29(1 Pt 2):171–6.
- McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S103–9; discussion 19–21.
- Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290:1029–30.
- Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis S, Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013.
- Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405.
- Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–6.
- Taubert D, Roesen R, Lehmann C, Jung N, Schomig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60.
- Flammer AJ, Hermann F, Sudano I, Spieker L, Hermann M, Cooper KA, et al. Dark chocolate improves coronary vasomotion and reduces platelet reactivity. Circulation. 2007;116:2376–82.
- Hollenberg NK, Fisher ND. Is it the dark in dark chocolate? Circulation. 2007;116:2360–2.
- Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, et al. (–) Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006; 103:1024–9.
- Baba S, Natsume M, Yasuda A, Nakamura Y, Tamura T, Osakabe N, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436–41.
- di Giuseppe R, Di Castelnuovo A, Centritto F, Zito F, De Curtis A, Costanzo S, et al. Regular consumption of dark chocolate is associated with low serum concentrations of C-reactive protein in a healthy Italian population. J Nutr. 2008;138:1939–45.
- Shiina Y, Funabashi N, Lee K, Toyoda T, Sekine T, Honjo S, et al. Relaxation effects of lavender aromatherapy improve coronary flow velocity reserve in healthy men evaluated by transthoracic Doppler echocardiography. Int J Cardiol. 2008;129:193–7.
- Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: the Stockholm Heart Epidemiology Program. J Intern Med. 2009;266:248–57.
- Buijsse B, Weikert C, Drogan D, Bergmann M, Boeing H. Chocolate consumption in relation to blood pressure and risk of cardiovascular disease in German adults. Eur Heart J. 2010;31:1616–23.
- Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20-year follow-up of 999 men. Hypertension. 1998;31:780–6.
- Desideri G, Kwik-Uribe C, Grassi D, Necozione S, Ghiadoni L, Mastroiacovo D, et al. Benefits in cognitive function, blood pressure, and insulin resistance through cocoa flavanol consumption in elderly subjects with mild cognitive impairment: the Cocoa, Cognition, and Aging (CoCoA) study. Hypertension. 2012;60:794–801.
- Mastroiacovo D, Kwik-Uribe C, Grassi D, Necozione S, Raffaele A, Pistacchio L, et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: the Cocoa, Cognition, and Aging (CoCoA) Study—a randomized controlled trial. Am J Clin Nutr. 2015;101:538–48.
- Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;343:d4488.
- Zomer E, Owen A, Magliano DJ, Liew D, Reid CM. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
- Esser D, Mars M, Oosterink E, Stalmach A, Muller M, Afman LA. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB J. 2014;28:1464–73.
- EFSA. Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No1924/2006. EFSA Journal. 2012;10:2809.
- EFSA. Scientific Opinion on the modification of the authorsation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No1924/2006 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA Journal. 2014;12:3654.
- Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–9.
- Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. New Engl J Med. 2006;355:2792–3.
- Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90.
- Kanner J, Harel S, Granit R. Betalains—a new class of dietary cationized antioxidants. J Agric Food Chem. 2001;49:5178–85.
- Hobbs DA, Kaffa N, George TW, Methven L, Lovegrove JA. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br J Nutr. 2012;108: 2066–74.
- Ghosh SM, Kapil V, Fuentes-Calvo I, Bubb KJ, Pearl V, Milsom AB, et al. Enhanced vasodilator activity of nitrite in hypertension: critical role for erythrocytic xanthine oxidoreductase and translational potential. Hypertension. 2013;61:1091–102.
- Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–7.
- Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. A single dose of beetroot juice enhances cycling performance in simulated altitude. Med Sci Sports Exerc. 2014;46:143–50.
- Kelly J, Vanhatalo A, Bailey SJ, Wylie LJ, Tucker C, List S, et al. Dietary nitrate supplementation: effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am J Physiol Regul Integr Comp Physiol. 2014;307:R920–30.
- Arnold JT, Oliver SJ, Lewis-Jones TM, Wylie LJ, Macdonald JH. Beetroot juice does not enhance altitude running performance in well-trained athletes. Appl Physiol Nutr Metab. 2015;40:590–5.
- Steffen M, Kuhle C, Hensrud D, Erwin PJ, Murad MH. The effect of coffee consumption on blood pressure and the development of hypertension: a systematic review and meta-analysis. J Hypertens. 2012; 30:2245–54.
- Watanabe T, Arai Y, Mitsui Y, Kusaura T, Okawa W, Kajihara Y, et al. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exp Hypertens. 2006;28:439–49.
- Yamaguchi T, Chikama A, Mori K, Watanabe T, Shioya Y, Katsuragi Y, et al. Hydroxyhydroquinone-free coffee: a double-blind, randomized controlled dose-response study of blood pressure. Nutr Metab Cardiovasc Dis. 2008;18:408–14.
- Revuelta-Iniesta R, Al-Dujaili EA. Consumption of green coffee reduces blood pressure and body composition by influencing 11beta-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. Biomed Res Int. 2014;2014:482704.
- Moreno-Luna R, Munoz-Hernandez R, Miranda ML, Costa AF, Jimenez-Jimenez L, Vallejo-Vaz AJ, et al. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am J Hypertens. 2012;25:1299–304.
- Perrinjaquet-Moccetti T, Busjahn A, Schmidlin C, Schmidt A, Bradl B, Aydogan C. Food supplementation with an olive (Olea europaea L.) leaf extract reduces blood pressure in borderline hypertensive monozygotic twins. Phytother Res. 2008;22:1239–42.
- Susalit E, Agus N, Effendi I, Tjandrawinata RR, Nofiarny D, Perrinjaquet-Moccetti T, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with Captopril. Phytomedicine. 2011;18:251–8.
- Ried K, Fakler P. Protective effect of lycopene on serum cholesterol and blood pressure: meta-analyses of intervention trials. Maturitas. 2011;68:299–310.
- Paran E, Novack V, Engelhard YN, Hazan-Halevy I. The effects of natural antioxidants from tomato extract in treated but uncontrolled hypertensive patients. Cardiovasc Drugs Ther. 2009;23:145–51.
- Petyaev IM, Dovgalevsky PY, Chalyk NE, Klochkov V, Kyle NH. Reduction in blood pressure and serum lipids by lycosome formulation of dark chocolate and lycopene in prehypertension. Food Sci Nutr. 2014;2:744–50.
- Ried K, Frank OR, Stocks NP, Fakler P, Sullivan T. Effect of garlic on blood pressure: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2008;8:13.
- Ried K, Frank OR, Stocks NP. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: a randomised controlled trial. Maturitas. 2010;67:144–50.
- Langsjoen PH, Langsjoen AM. Overview of the use of CoQ10 in cardiovascular disease. BioFactors. 1999;9:273–84.
- Ho MJ, Bellusci A, Wright JM. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst Rev. 2009;(4):CD007435.
- Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21:297–306.
- Maimoona A, Naeem I, Saddiqe Z, Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J Ethnopharmacol. 2011;133:261–77.
- Liu X, Wei J, Tan F, Zhou S, Wurthwein G, Rohdewald P. Pycnogenol, French maritime pine bark extract, improves endothelial function of hypertensive patients. Life Sci. 2004;74:855–62.
- Zibadi S, Rohdewald PJ, Park D, Watson RR. Reduction of cardiovascular risk factors in subjects with type 2 diabetes by Pycnogenol supplementation. Nutr Res. 2008;28:315–20.
- Hosseini S, Lee J, Sepulveda RT, Rohdewald P, Watson RR. A randomized, double-blind, placebo-controlled, prospective, 16 week crossover study to determine the role of Pycnogenol in modifying blood pressure in mildly hypertensive patients. Nutr Res. 2001;21:1251–60.
- Enseleit F, Sudano I, Periat D, Winnik S, Wolfrum M, Flammer AJ, et al. Effects of Pycnogenol on endothelial function in patients with stable coronary artery disease: a double-blind, randomized, placebo-controlled, cross-over study. Eur Heart J. 2012;33:1589–97.
- Rodella LF, Favero G, Foglio E, Rossini C, Castrezzati S, Lonati C, et al. Vascular endothelial cells and dysfunctions: role of melatonin. Front Biosci (Elite Ed). 2013;5:119–29.
- Grossman E, Laudon M, Zisapel N. Effect of melatonin on nocturnal blood pressure: meta-analysis of randomized controlled trials. Vasc Health Risk Manag. 2011;7:577–84.
- Scheer FA, Morris CJ, Garcia JI, Smales C, Kelly EE, Marks J, et al. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: a randomized controlled trial. Sleep. 2012;35:1395–402.
- Caligiuri SP, Edel AL, Aliani M, Pierce GN. Flaxseed for hypertension: implications for blood pressure regulation. Curr Hypertens Rep. 2014;16:499.
- Khalesi S, Irwin C, Schubert M. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J Nutr. 2015;145:758–65.
- Caligiuri SP, Aukema HM, Ravandi A, Guzman R, Dibrov E, Pierce GN. Flaxseed consumption reduces blood pressure in patients with hypertension by altering circulating oxylipins via an alpha-linolenic acid-induced inhibition of soluble epoxide hydrolase. Hypertension. 2014;64:53–9.
- Li H, Xia N, Forstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012;26:102–10.
- Mattison JA, Wang M, Bernier M, Zhang J, Park SS, Maudsley S, et al. Resveratrol prevents high fat/sucrose diet-induced central arterial wall inflammation and stiffening in nonhuman primates. Cell Metab. 2014;20:183–90.
- Poulsen MM, Vestergaard PF, Clasen BF, Radko Y, Christensen LP, Stodkilde-Jorgensen H, et al. High-dose resveratrol supplementation in obese men: an investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62:1186–95.
- Feringa HH, Laskey DA, Dickson JE, Coleman CI. The effect of grape seed extract on cardiovascular risk markers: a meta-analysis of randomized controlled trials. J Am Diet Assoc. 2011;111:1173–81.
- Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors - Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55.
- Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:823–36.
- Liu G, Mi XN, Zheng XX, Xu YL, Lu J, Huang XH. Effects of tea intake on blood pressure: a meta-analysis of randomised controlled trials. Br J Nutr. 2014;112:1043–54.
- de Jesus Romero-Prado MM, Curiel-Beltran JA, Miramontes-Espino MV, Cardona-Munoz EG, Rios-Arellano A, Balam-Salazar L. Dietary flavonoids added to pharmacological antihypertensive therapy are effective in improving blood pressure. Basic Clin Pharmacol Toxicol. 2015;117: 57–64.
- Cicero AF, Borghi C. Evidence of clinically relevant efficacy for dietary supplements and nutraceuticals. Curr Hypertens Rep. 2013;15:260–7.
