Abstract
Objective: Familial risks of glomerulonephritis (acute, chronic and unspecified glomerulonephritis) have not been studied. This study aims to determine the familial risks of glomerulonephritis.
Methods: Individuals born from1932 onwards diagnosed with glomerulonephritis (acute [n = 7011], chronic [n = 10,242] and unspecified glomerulonephritis [n = 5762]) were included. The familial risk (Standardized incidence ratio = SIR) was calculated for individuals whose parents/full-siblings were diagnosed with glomerulonephritis compared to those whose parents/full-siblings were not. The procedure was repeated for spouses. Familial concordant risk (same disease in proband and exposed relative) and discordant risk (different disease in proband and exposed relative) of glomerulonephritis were determined.
Results: Familial concordant risks (parents/full-sibling history) were: SIR = 3.57 (95% confidence interval, 2.77–4.53) for acute glomerulonephritis, SIR = 3.84 (3.37–4.36) for chronic glomerulonephritis and SIR = 3.75 (2.85–4.83) for unspecified glomerulonephritis. High familial risks were observed if two or more relatives were affected; the SIR was 209.83 (150.51–284.87) in individuals with at least one affected parent as well as one full-sibling. The spouse risk was only moderately increased (SIR = 1.53, 1.33–1.75).
Conclusions: Family history of glomerulonephritis is a strong predictor for glomerulonephritis, and is a potentially useful tool in clinical risk assessment. Our data emphasize the contribution of familial factors to the glomerulonephritis burden in the community.
The familial risks (full-sibling/parent history) of glomerulonephritis (acute, chronic and unspecified glomerulonephritis) have not been determined previously.
The familial risks of glomerulonephritis were increased among individuals with family history of acute, chronic or unspecified glomerulonephritis.
The familial risks of glomerulonephritis were slightly increased among spouses indicating a modest non-genetic contribution.
Very high familial risks were observed in multiplex families, i.e. with one or more affected first-degree relatives.
Key Messages
Introduction
Kidney diseases are a global health challenge among all societies across the world with high impact on morbidity and mortality (Citation1,Citation2). With an increasing global population and a higher prevalence of kidney diseases, the need for a more effective prevention and cost-effective approach to tackle this condition is necessary (Citation3). Glomerulonephritis is a common cause of end-stage kidney failure worldwide (Citation4). The most common forms of glomerulonephritis in adults are IgA nephropathy, focal and segmental glomerulosclerosis, and vasculitis (Citation4). In children the most common forms are minimal change disease, focal and segmental glomerulosclerosis, lupus nephritis- and IgA nephropathy (Citation4).
Several single genes have been identified in patients with glomerular diseases, such as steroid-resistant nephrotic syndrome and focal segmental glomerulosclerosis (Citation5). However, single-gene disorders are rare diseases. Our knowledge regarding common polygenic variants involvement in the familial risks of glomerulonephritis is uncertain, though common variants have been associated with other kidney disorders. These include as variants in the UMOD, PRKAG2, APOL1 and MYH9 genes (Citation5–9). Several studies have recognized that glomerulonephritis may run in families (Citation10–12). However, the familial risks of these diseases remain to be determined.
Knowledge of familial risks could be of clinical use in identifying individuals with an increased risk for glomerulonephritis. Such knowledge may help clinicians to select high-risk individuals for disease screening. The aim of the present study was to determine the importance of familial factors to the glomerulonephritis burden in the community. We hypothesized that glomerulonephritis in parents/full-siblings is associated with an increased risk of glomerulonephritis in offspring/full-siblings. In addition, we determined spouse risks in order to reflect the non-genetic adult familial risks.
Material and methods
Study design
The dataset used in this study was constructed by linking several national Swedish registers provided by the Swedish Government-Owned Statistics Bureau, Statistics Sweden, and the National Board of Health and Welfare (Citation13–17). The Swedish multigenerational register contains information on family relationships for index persons born in Sweden from 1932 onwards. Individuals aged 0–78 years constituted the present study population. Linkages were made to National Census data (in order to ascertain individual-level socioeconomic status), the Swedish cause of death register (1964–2010), the Swedish outpatient care register (2001–2010), and the Swedish hospital discharge register (1964–2010); the last of which records complete nationwide data of hospitalizations and hospital diagnoses since 1987. All linkages were performed using the national personal identification number that is assigned to each resident in Sweden for their lifetime. This number was replaced by a serial number in order to preserve anonymity (Citation18). The serial numbers were used to check that each individual was entered only once (for his or her first main or secondary diagnosis of glomerulonephritis). Approximately, 8 million individuals and their biological parents (3.8 million families) were included in the database; the oldest (born in 1932) were 78 years at the end of the follow-up period, which ran from 1964 to 2010.
Predictor and outcome variables
The predictor variable was family history (in a full-sibling and/or parent) of glomerulonephritis (defined below) between 1964 and 2010. Family history was only based on registry records (Multi-generation register), which eliminates recall bias from the proband. Separate risks were determined for parental and full-sibling history of glomerulonephritis. Full siblings were defined from the Multi-generation register by having the same mother and father, and the term sibling in this paper, refers to full-sibling. Thus, individuals without any full-sibling alive any time during the follow-up period between 1987 and 2010 were excluded in the analysis of familial sibling risks. Risk for spouses was also calculated. Spouses were defined as individuals older than 25 years with a common oldest child. Thus, only spouses with children were included in the spouse analysis. The outcome variable was first main or secondary diagnosis of glomerulonephritis (acute, chronic and unspecified glomerulonephritis) in the Swedish hospital discharge register or the Swedish outpatient care register. Acute glomerulonephritis was defined by the following ICD codes (international classification of diseases): 590 (ICD-7), 580 (ICD-8-9) and N00-N01 (ICD-10). Unspecified glomerulonephritis was defined by the following ICD codes: 593 (ICD-7), 583 (ICD-8-9) and N05 (ICD-10). Chronic glomerulonephritis was defined by the following ICD codes: 592 (ICD-7), 582(ICD-8-9) and N03 (ICD-10). Main (the main cause for hospitalization) and secondary diagnoses were considered.
Individual variables included in the analysis
The following variables were included in the analysis: (1) Gender: males or females; (2) Age: age at diagnosis was categorized into five-year groups; (3) Time period: the follow-up period was divided into five-year intervals in order to adjust for changes in hospitalization rates over time; (4) Socioeconomic status: socioeconomic status was defined by occupation for both males and females, which was divided into six groups: (1) farmers, (2) blue-collar workers, (3) white-collar workers, (4) professionals, (5) self-employed workers and (6) others (economically inactive individuals including unemployed persons and homemakers); (5) Geographic region of residence: to allow adjustment for regional differences in hospitalization rates, geographic region of residence was divided into three groups: (1) Southern Sweden; (2) large cities; and (3) Northern Sweden. Large cities were defined as municipalities with a population of >200,000 and comprised the three largest cities in Sweden: Stockholm, Gothenburg and Malmö.
Statistical analysis
A previously described method was used (Citation19) for the analysis of familial risks of glomerulonephritis. The method is described in detail by Hemminki et al. (Citation20) and takes into account clustering within families, since it is based on complete ascertainment of sibships in affected individuals. Person-years at risk (i.e. the number of persons at risk multiplied by the time at risk) were calculated from the start of the follow-up on 1 January 1964 until diagnosis for glomerulonephritis, death, emigration, or the end of the follow-up (31 December 2010) (Citation13). Age-adjusted incidence rates were calculated for the whole follow-up period, divided into five-year periods (Citation21). Standardized incidence ratios (SIRs) were used to measure the relative risk of glomerulonephritis in individuals with one or more parents with a history of glomerulonephritis, compared with individuals with parents without a history of glomerulonephritis. Similar calculations were performed separately for full-siblings.
The familial SIRs were calculated as the ratio of observed (O) and expected (E) numbers of glomerulonephritis cases using the indirect standardization method:
where,
denotes the total observed number of cases in the study group; E* (the expected number of cases) is calculated by applying stratum-specific standard incidence rates (λj*) obtained from the reference group to the stratum-specific person-years of risk (nj) for the study group; oj represents the observed number of cases that the cohort subjects contribute to the jth stratum; and J represents the strata defined by cross-classification of the following adjustment variables: age (five-year groups), sex, socioeconomic status, time period (five-year groups), and geographic region of residence. 95% confidence intervals (95% CIs) were calculated assuming a Poisson distribution (Citation21). Power calculation was not performed, as the study population was a nationwide cohort.
Familial SIRs for males were compared directly with those for females through calculation of SIR ratios according to the method described by Breslow and Day (Citation21). The SIR ratios represent the relative risks for familial glomerulonephritis in males compared with females. SIR ratios have the same interpretation as the relative risk parameters estimated in case-control studies. They represent the ratios of age-specific rates for different exposure categories.
Data values are accurate to two decimals places. All analyses were performed using SAS version 9.3 (Institute, Cary, NC).
Ethical considerations
Statistics Sweden and the National Board of Health and Welfare maintain the nationwide registers used in the present study. This study was approved by the Ethics Committee at Lund University (approval number 409/2008 Lund with complementary approvals dated 1 September 2009 and 22 January 2010) and the recommendations of the Declaration of Helsinki were complied with. The ethics committee waived informed consent as a requirement.
Results
Study population
We analyzed the familial risks of glomerulonephritis (acute, chronic and unspecified glomerulonephritis) in the full-siblings/offspring (aged 0-78 years) of 8,187,887 individuals assessed in the registers for a clinical diagnosis of glomerulonephritis between 1964 and 2010 in Sweden. A total of 23,015 individuals were diagnosed with glomerulonephritis: 61% (14,009) males and 39% (9006) females (). Individuals with acute glomerulonephritis 30.5% (n = 7011), chronic glomerulonephritis 44.5% (n = 10,242) and unspecified glomerulonephritis 25% (n = 5762) were included. Many individuals with glomerulonephritis were diagnosed at a young age.
Table 1. Characterstics of Swedish patients with glomerulonephritis born 1932 until 2010.
Familial risks of glomerulonephritis
Familial risks of glomerulonephritis according to disease subtypes are presented in . Increased risks were observed for paternal, maternal, paternal and full-sibling history of glomerulonephritis. The familial risks were highest for chronic glomerulonephritis in all lines; the SIR for chronic glomerulonephritis and parental history of any glomerulonephritis was 3.18 (95% CI 2.81–3.58). The familial SIR was 1.64 (95% CI 1.32–2.01) for acute glomerulonephritis and 2.54 (95% CI 2.11–3.05) for unspecified glomerulonephritis. Familial risks of glomerulonephritis were increased both in females and males ().
Table 2. Familial risks of glomerulonephritis (acute, chronic and unspecified) according to relatedness.
Individuals with an affected full-sibling with any type of glomerulonephritis had a higher risk of chronic glomerulonephritis (SIR = 3.73, 95% CI 3.26–4.26). The sibling risks were also increased for acute glomerulonephritis (SIR = 2.93, 95% CI 2.38–3.56) and unspecified glomerulonephritis (SIR = 3.37, 95% CI 2.75–4.10) (). The sibling risks were generally higher than the parent-offspring risks.
Familial risks of glomerulonephritis in different ages
In , familial risks are presented according to the relative affected (parents or full-siblings) at different ages. Familial risks were increased in all age groups. The parental risk was highest for individuals aged 30–39 years (SIR = 3.38, 95% CI 2.76–4.10). The sibling risk was highest for individuals aged 20–29 years (SIR = 4.74, 95% CI 3.85–5.78).
Table 3. Age- and sex-stratified familial risks of glomerulonephritis.
Gender differences
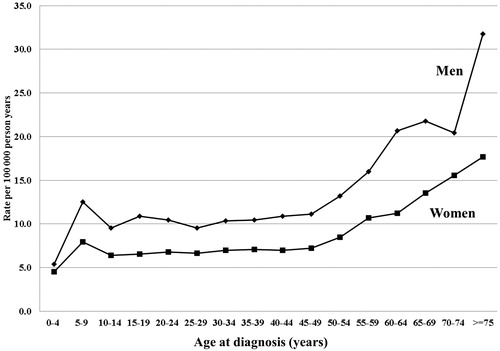
We calculated gender differences for glomerulonephritis by estimating family risk ratios and incidence rate ratios between males and females. The SIR ratio (male/female) was 1.15 (95% CI 0.95–1.35), p = 0.1553. The incidence rate ratio (male/female) was 1.52 (95% CI 1.47–1.56), p < 0.001 (calculations were based on an incidence rate of 10.9 per 1,00,000 person years for males and 7.2 per 1,00,000 person years for females. Age- and gender-specific incidence rates of glomerulonephritis are presented in and .
Discordant familial risks of glomerulonephritis
Concordant (same disease in proband and offspring) and discordant (different disease in proband and offspring) familial risks are presented in . There were increased familial risks for individuals with affected family members (family history) of all types of glomerulonephritis. The concordant familial SIR for acute glomerulonephritis among individuals with a family history of acute glomerulonephritis was 3.57 (95% CI 2.77–4.53). The corresponding SIRs were 3.84 (95% CI 3.37–4.36), for chronic glomerulonephritis and 3.75 (95% CI 2.85–4.83) for unspecified glomerulonephritis. The discordant familial risks were also increased. A family history of any glomerulonephritis increased the risk of any glomerulonephritis disease; the SIR was 2.92 (95% CI 2.72–3.14).
Table 4. Concordant and discordant familial risks (parent and/or full-sibling) of glomerulonephritis.
Multiplex families
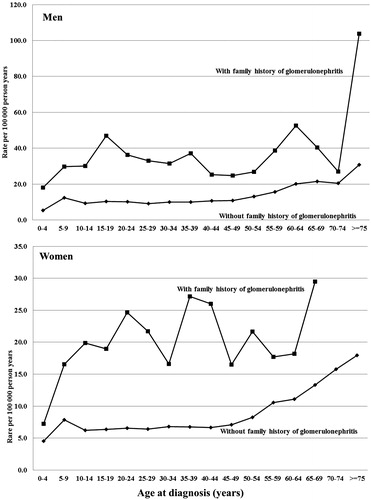
Familial SIRs for glomerulonephritis according to number and type of probands are summarized in . The SIR for glomerulonephritis in individuals with one affected parent was 2.54 (95% CI, 2.31–2.78). The SIR for glomerulonephritis when both parents were affected was 6.40 (95% CI, 1.67–16.55).
Table 5. Familial risk of glomerulonephritis according to number of affected relatives.
When at least one parent and one full-sibling were affected, the SIR was 209.83 (95% CI, 150.51–284.87). The SIR was 263.16 (95% CI, 173.25–383.35) when two full-siblings were affected. The familial SIR was 3.24 (95% CI, 2.93–3.58) for individuals with one affected full-sibling, and 263.16 (95% CI 173.25–383.35) for those with two affected full-siblings.
Test for the extent of the shared non-genetic familial contribution
Two kinds of analyses were performed to test for the extent of environmental sharing in the observed predictor of glomerulonephritis. Familial risks were calculated for spouses diagnosed with glomerulonephritis. The overall familial risk of any glomerulonephritis (acute, chronic and unspecified glomerulonephritis) was only modestly increased in spouses among males and females; the SIR was 1.53 (95% CI 1.33–1.75). Second, SIRs for full-sibling pairs (sib-pairs) according to age difference were calculated (). Siblings with a difference in age of less than five years had a SIR of 3.62 (95% CI 3.20–4.08) compared with 2.63 (95% CI 2.19–3.13) for those with a difference of at least five years (). Moreover, for chronic, unspecified and acute glomerulonephritis the familial risks tended to be highest among siblings with an age difference of less than five years, although the confidence intervals overlapped.
Table 6. Familial risk of glomerulonephritis in sib-pairs (full-siblings) by age difference.
Discussion
Statement of new findings
The present study is the first nationwide follow-up study to evaluate the familial risks of glomerulonephritis (acute, chronic and unspecified glomerulonephritis) among offspring/full-siblings and spouses of affected individuals. Our results indicate that family history (parents and/or full-siblings) of glomerulonephritis is a strong predictor for glomerulonephritis. This is in agreement that causative mutations have been identified in patients with glomerular disease (Citation6). The present results also indicate that other familial factors are important for glomerulonephritis in both males and females. Very high risks were noted among multiplex families, suggesting strong genetic factor segregation in these rare families (). Moreover, unique familial (environmental or genetic) factors may predispose individuals to glomerulonephritis. The familial concordant risks were high for chronic glomerulonephritis. The familial concordant risks for acute and unspecified glomerulonephritis were somewhat lower than those for chronic glomerulonephritis. The present study indicates that familial factors are of importance in acute, chronic and unspecified glomerulonephritis ( and ). The familial risk for unspecified glomerulonephritis was somewhere between that of the acute and chronic groups; the unspecified glomerulonephritis group may thus comprise a mixture of acute and chronic glomerulonephritis. Our study confirms previous studies that have recognized that glomerulonephritis run in families (Citation10–12).
Testing genetic hypothesis
Spouses are genetically unrelated, but share adult environments and similar sociodemographic characteristics (Citation22). Their family histories are thus matched in terms of many of the factors one might wish to control for in testing a genetic hypothesis. Spouse risks were low compared to familial risks in first-degree relatives. The spouse risk for glomerulonephritis was much lower than the sibling or offspring risks, suggesting that the familial risks in offspring and full-siblings to a large extent are genetic. The increased spouse risk may be related to shared familial environmental exposures, such as smoking, alcohol, diet, exercise habits and infections in adulthood (Citation22). There was also a tendency for higher familial risks for glomerulonephritis in siblings with a difference in age of less than five years, which further suggests a non-genetic effect of shared familial environments. The exposure for environmental factors in different generations may vary. Such environmental factors could be infections, food and certain chemicals (Citation23). However, the very high risk in multiplex families indicates a strong genetic cause (Citation24). Another possible hypothesis for the tendency for higher sibling than parent-offspring risk could be due to recessive genes (Citation6).
The Swedish hospital discharge register contains no information about diagnostic procedures (e.g. kidney biopsies), which is a limitation. Moreover, the use of ICD codes is limited given the distinction of acute, chronic and unspecified glomerulonephritis. This distinction largely has a historic basis and relates to a time when post-streptococcal glomerulonephritis was relatively common. Nowadays, many nephrologists have largely abandoned distinguishing between acute and chronic glomerulonephritis (Citation4). This is reflected by the relative high rate of acute glomerulonephritis of 30.5% in the present study (Citation4). We could not differentiate between primary and secondary glomerulonephritis. The lack of risk factors is a potential confounder, but it is probably a non-differential bias regarding familial risks. The validity of ICD codes for kidney disease has not been examined. However, the Swedish hospital discharge register has been extensively validated and its overall diagnostic validity is around 85-95% for most diseases (Citation13,Citation16). Moreover, specialist doctors in hospital care made the diagnosis. A good concordance of 89% was found between hospital discharge diagnoses and the underlying causes of death of those who were hospitalized and later died under dramatic conditions (Citation25). As it is possible that the diagnostic accuracy could have varied between geographic regions, we adjusted for geographic region in order to minimize this possible bias. The higher risk associated with a family history might also, to a certain degree, be caused by detection bias/activity as well as a lower threshold for seeking medical help. However, the modest spouse risk suggests that this potential bias is modest regarding familial risks. The lack of nationwide data regarding family history before 1987 is also most likely a non-differential bias regarding family history of VTE. A most likely non-differential bias regarding familial risks is also that cases in probands and relatives before 1964 were unknown. Another limitation is that we had no data on life-style related factors, such as body mass index (BMI), smoking and diet, because it would be unrealistic to gather such data for an entire national population. However, we did adjust for socioeconomic status, which is associated with many life-style factors, such as smoking. Another limitation is that some findings may have been caused by chance because of the multiple comparisons performed.
We calculated gender differences for glomerulonephritis by incidence rate ratios and family risk ratios between males and females. There were no differences in the familial risk between males and females (SIR ratio). However, glomerulonephritis was more common in males compared to females (incidence rate ratio).
Strengths of the study include complete nationwide coverage from 1987 in a country with high standards of diagnosis surveilled by the Swedish National Board of Health and Welfare, with diagnoses often being made by specialists during extended examinations in clinics. Thus, our data reflect the total impact of a familial history of glomerulonephritis in the whole population of Sweden. There is also an increasing number of Swedish National Quality Registers (around 100 registers) that contain individualized data concerning patient problems, medical interventions, and outcomes after treatment (Citation26). Another important strength of our study is that it was based on nationwide registers and was thus free of recall bias. Selection bias was also minimized. The Swedish multigenerational register and the Swedish hospital discharge register are validated data sources that have been proven to be reliable in the study of many diseases (Citation14–17). Data in our dataset are almost 100% complete (Citation15).
Finally, although our study was limited to Sweden, the results from Swedish nationwide family studies are likely to be valid for Caucasian populations in Europe and the United States (Citation17).
Conclusion
In summary, the present study found indications of strong familial aggregation in acute, chronic and unspecified glomerulonephritis. Familial adult non-genetic contributions are suggested to be moderate. Our data not only emphasize the contribution of familial factors to the glomerulonephritis burden in the community, but also suggest a causal relation of genetic factors to the disease process. Our findings suggest that information should be collected on parental/sibling glomerulonephritis as part of the family history to help identify persons at risk for glomerulonephritis.
Funding information
This work was supported by grants awarded to Dr Bengt Zöller by the Swedish Heart-Lung Foundation, the Swedish Research Council and ALF funding from Region Skåne. Grants were awarded to Dr Kristina Sundquist by ALF funding from Region Skåne, the Swedish Research Council, and grants awarded to Dr Jan Sundquist by the King Gustaf V and Queen Victoria’s Foundation of Freemasons. Statistics Sweden and the National Board of Health and Welfare maintain the registers used in the present study.
Acknowledgements
The authors wish to thank the CPF’s Science Editor Patrick Reilly for his useful comments on the text.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–80.
- Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72:247–59.
- Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Cores J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005;67:2089–100.
- Chadban SJ, Atkins RC. Glomerulonephritis. Lancet. 2005;365:1797–806.
- Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009;41:712–17.
- Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287–95.
- Drawz PE, Sedor JR. The genetics of common kidney disease: a pathway toward clinical relevance. Nat Rev Nephrol. 2011;7:458–68.
- Köttgen A. Genome-wide association studies in nephrology research. Am J Kidney Dis. 2010;56:743–58.
- Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–92.
- Rambausek MH, Waldherr R, Ritz E. Immunogenetic findings in glomerulonephritis. Kidney Int Suppl. 1993;39:S3–8.
- Izzy C, Sanna-Cherchi S, Prati E, Belleri R, Remedio A, Tardando R, et al. Familial aggregation of primary glomerulonephritis in an Italian population isolate: Valtrompia study. Kidney Int. 2006;69:1033–40.
- Scolari F, Amoroso A, Savoldi S, Prati E, Scaini P, Manganoni A, et al. Familial occurrence of primary glomerulonephritis: evidence for a role of genetic factors. Nephrol Dial Transplant. 1992;7:587–96.
- National Board of Health and Welfare. Validity of the diagnoses from the Swedish In-Care Register 1987 and 1995 [in Swedish]. Stockholm, Sweden: Epidemiologiskt Centrum, Socialstyrelsen, 2000.
- Rosen M, Hakulinen T. Use of disease registers. In: Ahrens W, Pigeot I, eds. Handbook of epidemiology, pp. 231–52. Berlin: Springer-Verlag, 2005.
- Ekbom A. The Swedish Multi-generation Register. Methods Mol Biol. 2011;675:215–20.
- Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- Zöller B. Nationwide family studies of cardiovascular diseases – clinical and genetic implications of family history. EMJ Cardiol. 2013;1:102–13.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–67.
- Zöller B, Li X, Sundquist J, Sundquist K. Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation. 2011;124:1012–20.
- Hemminki K, Vaittinen P, Dong C, Easton D. Sibling risks in cancer: clues to recessive or X-linked genes? Br J Cancer. 2001;84:388–91.
- Breslow NE, Day NE. Statistical methods in cancer research. The design and analysis of cohort studies. IARC Sci Publ. 1987;2:1–406.
- Lawlor DA, Mishra GD. Family matters: designing, analysing and understanding family based studies in life course epidemiology (A life course approach to adult health). pp. 4–5. New York: Oxford University Press, 2009.
- Segelmark M, Hellmark T. Autoimmune kidney diseases. Autoimmun Rev. 2010;9:A366–71.
- Burton PR, Tobin MD, Hopper JL. Key concepts in genetic epidemiology. Lancet. 2005;366:941–51.
- Johansson LA, Westerling R. Comparing Swedish hospital discharge records with death certificates: implications for mortality statistics. Int J Epidemiol. 2000;29:495–502.
- Nilsson E, Orwelius L, Kristenson M. Patient-reported outcomes in the Swedish National Quality Registers. J Intern Med. 2016;279:141–53.