Abstract
Aims. To investigate the medium-term effects of estrogen plus progesterone therapy (EPT) on vascular reactivity, endothelial function and hemodynamic responses in 20 hypertensive postmenopausal women. Methods. This randomized, double-blind, cross-over, placebo-controlled study investigates the effect of 6 months of EPT (conjugated equine estrogen plus medroxyprogesterone). Blood pressure (office and ambulatory), heart rate and heart rate variability (HRV) were measured at baseline and following EPT/placebo treatment. In eight women, we used a wire-myograph to assess endothelial function and contractile response of subcutaneous arteries to transmural nerve stimulation (TNS) and exogenous noradrenaline. Results. EPT decreased vascular reactivity to cumulative TNS compared with baseline (p<0.01) and placebo (p<0.05). Moreover, EPT diminished sensitivity to exogenous noradrenaline (p<0.05). Although EPT reinforced response to acetylcholine, we observed no difference in maximal relaxation induced by substance P or acetylcholine. EPT did not affect ambulatory blood pressure, heart rate or HRV. Conclusions. Oral combined medium-term EPT reduces adrenergic reactivity in subcutaneous arteries from treated hypertensive postmenopausal women. EPT might act postjunctionally at the adrenergic vascular receptor level. In the present study, EPT neither reduces sympathetic activity nor increases vagal tone, and thus does not support an effect on the central hemodynamic system.
Introduction
Blood pressure (BP) increases with age, and hypertension is an independent and strong risk factor for cardiovascular events (Citation1,Citation2). Epidemiological studies suggest that hormone therapy (HT) protects against cardiovascular disease (Citation3) and menopause per se likely results in a sharper increased BP (Citation4). However, large clinical trials have not shown positive effects of HT on BP (Citation5) or cardiovascular outcomes in healthy normotensive postmenopausal women or postmenopausal women with established coronary heart disease (Citation6,Citation7). Some studies have reported that HT lowers BP (Citation8), possibly suggesting a positive cardiovascular effect of estrogen in hypertensive but otherwise healthy postmenopausal women.
Hemodynamic response to stress correlates independently to target organ damage (Citation9). Borderline-hypertensive and hypertensive humans and animals show greater cardiovascular reactivity to different stress stimuli compared with normotensive controls (Citation10). Interestingly, female sex hormones seem to prevent this harmful response of the autonomic nervous system (ANS) (Citation11,Citation12).
Estrogen's cardioprotective effect may partially result from its influence on the sympathoadrenergic system (Citation13). Indeed, estrogen therapy (ET) and estrogen plus progestogen therapy (EPT) in healthy postmenopausal women alters autonomic cardiovascular function towards increased vagal activity (Citation14). We reported previously that 17β-estradiol reduces adrenergic reactivity in the resistance arteries of hypertensive rats (Citation15). However, estrogen's effect on human resistance arteries in hypertensive patients remains poorly understood.
In the present hypothesis-generating study, we specifically aimed to investigate the effect of medium-term EPT on the ANS and vascular reactivity in hypertensive but otherwise healthy, postmenopausal women. We evaluated the effect of EPT on mental stress reaction, ambulatory BP, heart rate (HR), and spectral analysis of heart rate variability (HRV) as well as adrenergic reactivity and endothelial function in small subcutaneous resistance arteries.
Materials and Methods
Subjects
This study was approved by the Ethics Committee at Göteborg University and performed in accordance with the Helsinki Declaration of Health. Twenty hypertensive postmenopausal women (mean age 56 years; range 53–61 years) were recruited by an advertisement in the local newspaper in Göteborg, Sweden. Menstrual bleeding had ceased for at least 2 years, and none of the participants had received HT for at least 3 months prior to the study. Subjects were treated with one to four antihypertensive drugs (), and we aimed to maintain office BP at the same level throughout the study. All subjects were non-smokers and had no history of thromboembolic disease, diabetes, hyperlipidemia, alcohol abuse, or malignancy. Before enrolling in the study, all subjects performed normal ECG, two BP measurements ≤170/100 mmHg, normal measurements of p-APC-resistance, p-protein C and S, and normal mammography, physical and gynecological examinations.
Table I. Anti hypertensive drugs in 20 hypertensive postmenopausal women at baseline and after 6 months of treatment with placebo and Premelle®.
Study design
The study was performed as a randomized, double-blind, cross-over and placebo-controlled study that tested conjugated equine estrogen plus medroxyprogesterone (Premelle®). Prior to each investigation (baseline, 6 months and 12 months later), subjects entered our laboratory after fasting (12 h). Subjects rested alone in a quiet room for 30 min before we recorded supine office BP. We collected blood samples for analysis of s-estradiol, s-testosterone and s-SHBG. Subjects then participated in a 30-min mental stress test (mental arithmetic) while we monitored BP and HR at 1-min intervals. Seventeen women were submitted to gluteal biopsy, covering 1×0.5×1 cm of subcutaneous tissue, under local anaesthesia (Xylocain 10% and adrenaline 1%). We mounted small subcutaneous arteries in a wire-myograph and studied the effects of EPT on vascular reactivity and endothelial function. Finally, we monitored ambulatory BP for 24 h [Spacelab 90202) (3×/h (06:00 h to 24:00 h) and 2×/h (00:00 h to 06:00 h)] as well as HR while subjects were outside the hospital. To further analyze the effect of EPT on autonomic control, we performed time domain and frequency domain analysis of HRV.
Stress experiment
Subjects performed a three-part mental arithmetic test while we monitored BP and HR at 1-min intervals (Spacelab 90202, Redmond, WA, USA). In the first segment of the test, subjects rested in a supine position while a laboratory nurse recorded BP and HR. During the second segment, we asked subjects to subtract sequentially the number seven from 100 (e.g. 100-7 = 93; 93-7 = 86; etc.) apace with a metronome and distracted by a bell. In the final segment, we continued monitoring BP and HR at 1-min intervals while subjects rested in a quiet room.
Spectral analysis
Beginning at 11:00 h, subjects underwent Holter monitoring (24 h) that used a three-channel amplitude-modulated tape recorder (ASPECT Holter System, Danica Biomedical AB, Borlänge, Sweden) with three leads (modified inferior, CM1 and CM5). The subjects were told to maintain their normal daily activities during this period. Tape analysis was accomplished with the ASPECT Holter System (Barlett) analysis programme, and subsequent HRV was measured by the Welsh programme. Electrocardiogram strips showing the interval of R-R distribution were visually checked and the frequency histogram of normal R-R intervals was displayed, excluding cycles outside 80-120% of the preceding R-R intervals, as well as artefacts, premature beats and postextrasystolic pauses. HRV time-domain measurements, calculated from 24-h electrocardio-graphic recordings, included the mean of all normal R-R intervals (mean-NN); standard deviation of all normal R-R intervals (SDNN); standard deviation of averaged R-R intervals of all 5-min segments (SDANN); the square root of the mean of the squared differences between adjacent successive R-R intervals (rMSSD); and the percentage of difference between adjacent normal R-R intervals >50 ms (pNN50). We computed spectral power measurements using Welsh (FFT) transform analysis, calculating total oscillatory power (TotP; 0.003–0.400) and identifying three subsets of frequency domain: low frequency power (LF; 0.040–0.150 Hz); high frequency power (HF; 0.150– 0.400 Hz); and very low frequency power (VLF; 0.003– 0.040 Hz). Spectral power was quantified and squared in the three frequency bandwidths, stating the average spectral power in each frequency in absolute values (ms2) and in normalized units (NU): LF NUs=LF power/(TotP-VLF power)×100. HF NU=HF power/ (TotP-VLF power)×100. Furthermore, the ratio of LF to HF power (LF/HF) was calculated.
Myograph experiments
Subcutaneous fat biopsies were placed in cold physiological saline solution (PSS) and kept on ice during the mounting procedure. The arteries (2 mm long; 464±33 μm internal diameter) were dissected free from connective tissue, threaded onto two fine stainless steel wires (40 μm diameter), and mounted in a Multi Myograph 610M (Danish Myo Technology, Aarhus, Denmark). Following equilibration (30 min), we set the vessels to a normalized internal circumference 0.9 times the estimated circumference they would maintain if relaxed and exposed to a transmural pressure of 100 mmHg, i.e. the optimal level for active force and isometric artery wall tension are recorded at well-defined internal circumferences (Citation16). The organ bath contained PSS (7 ml) equilibrated with 5% CO2 in air (pH 7.4) at 37°C. Before initiating experiments, the vessels were equilibrated again (30 min) and maximally contracted with nora-drenaline (NA; 10 μmol/l) and potassium salt (KCl; 125 mmol/l). Only 17 subjects were positive to take part in the substudy where biopsy was performed. Complete data was collected from eight of these 17 subjects: one subject left the study, and we encountered difficulty in finding appropriate vessels in all three biopsies (baseline, 6 months and 12 months) in eight other subjects, perhaps related to rarefaction.
We performed transmural nerve stimulation (TNS) by electrical field stimulation, passing constant current pulses (85 mA) and alternating polarity (0.1 ms) between two platinum electrodes placed on either side of the vessel segment in the presence of cocaine (1 μmol/l) and propranolol (1 μmol/l). Tetrodotoxin (0.1 μmol/l) has been shown to block ensuing contraction and was therefore considered neurogenic (Citation15). We used a cumulative frequency-response relationship to TNS (0.02-16 Hz, approximately 30-60 s duration), as purinergic components diminish progressively with stimulation, isolating the adrenergic component of contraction.
In a parallel set of vessels, we obtained a cumulative concentration-response relationship to applied NA (0.02-10 μmol/l), in presence of propranolol (1 μmol/l) and KCl (10-125 mmol/l), increasing the concentration two-fold every 2 min. Endothelial function was evaluated by the response to acetylcho-line (ACh; 10−9 to 3×10−6 mol/l) and substance P (SP; 10−12 to 3×10−8 mol/l) on NA-precontracted vessels (maximal contraction) in random order. The vessels were incubated with N(ω)-nitro-L-arginine (L-NNA) (300 μmol/l) for 30 min and the dose-response curves to ACh and SP were repeated. The dose-response curve to ACh was repeated in the presence of the endothelial-derived hyperpolarization factor (EDHF) blockers, charybdotoxin (0.1 μmol/l) and apamin (0.7 μmol/l) (Citation17).
Solutions and drugs
Subjects ingested Premelle® (conjugated oestrogen USP 0.625 mg and medroxyprogesteronacetat 5 mg) and placebo tablets daily (Organon AB, Fiskhamnsgatan 6A, S-414 58 Göteborg, Sweden). NA ([−]-arterenol), ACh, SP, cocaine, propranolol, L-NNA, charybdotoxin and apamin were purchased from Sigma (St Louis, MO, USA). PSS composition was 119 mmol/l NaCl, 25 mmol/l NaHCO3, 5.5 mmol/l glucose, 4.7 mmol/l KCl, 2.5 mmol/l CaCl2, 1.18 mmol/l KH2PO4, 1.17 mmol/l MgSO4, 0.026 mmol/l ethylenediamine tetraacetic acid. We administered potassium salt by applying the salt solution where NaCl had been replaced partially by an equimolar amount of KCl. Stock solutions of applied drugs were dissolved in distilled water and diluted in PSS.
Statistics
Subjects were used as their own controls.
We used analysis of variance (ANOVA) to analyze repeated measures of office BP, biochemical parameters, HRV and averaged values from all three segments of the mental stress test. Because of skewed distribution, spectral power was transformed into its natural logarithm (Ln) (Citation18).
Ambulatory monitored data, calculated as 1-h means of HR, systolic blood pressure (SBP) and diastolic blood pressure (DBP), were analyzed for repeated measurements by two-way ANOVA, taking into account subjects, treatment and time. Day was defined as 06:00 h to 23:00 h, and night as 23:00 h to 06:00 h.
We used non-linear regression (Graph Pad Systems, San Diego, CA, USA) to analyze concentration-response relationships, fitting the curves to individual concentration-response data based on the relationship E=Emax AP(AP+EC50P)−1, where E represents the response obtained for a given concentration A, Emax is the maximally attainable response, EC50 is the concentration required for half-maximal effect, and exponent P represents the slope of the relationship (Hill coefficient). TNS-mediated contraction is presented as percent of maximal NA-induced contraction. NA- and KCl-mediated contractions are expressed as percent of Emax of each agonist, respectively. ACh- and SP-mediated relaxation was calculated as percent change of maximal NA-induced contraction. Statistical analysis was performed by ANOVA for repeated measures and two-way-repeated-measures ANOVA, using agonist concentration and isometric wall tension as a within-subject factor and treatment as a between-subject factor. p<0.05 is regarded significant. Values are given as mean±SEM.
Results
Subjects showed no significant differences in body mass index (BMI) or testosterone levels after treatment with placebo or EPT (). Compared with baseline and placebo, s-estradiol and s-SHBG increased significantly after EPT treatment (). Subjects who were submitted to gluteal biopsy did not differ with respect to age or BMI compared with subjects not taking part in this substudy.
Table II. Body mass index (BMI), office blood pressure and biochemical descriptions in 20 hypertensive postmenopausal women at baseline and in response to 6 months of treatment with placebo and 6 months of treatment with Premelle®.
Blood pressure
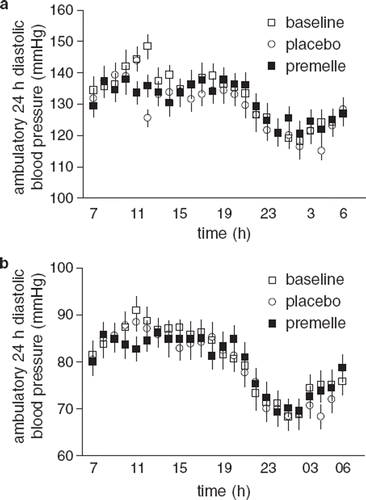
EPT did not alter office BP (), and we observed no significant effect on diastolic 24-h ambulatory recordings (). However, we detected a significant interaction (treatment×time, p<0.05) of the 24-h ambulatory SBP curve, which flattened during the morning hours following EPT compared with baseline and placebo (). Subjects who were submitted to gluteal biopsy did not differ regarding office SBP or DBP at baseline.
Heart rate
We observed a significant interaction (treatment×time, p<0.001) between baseline, placebo, and EPT during the 24-h HR ambulatory recording, possibly related to increased HR after placebo treatment during the morning hours. Since we observed no difference compared with baseline, it appears that EPT does not influence HR.
Stress test
During mental stress SBP, DBP and HR increased significantly compared with pre-stress conditions, irrespective of treatment (p<0.001). SBP and DBP increased significantly at baseline in response to stress compared with placebo treatment and EPT (p<0.05). However no difference in SBP, DBP or HR was seen between placebo and EPT.
Frequency domain analysis
We observed no statistical differences in any time domain or spectral analysis of HRV measurements between baseline, placebo, and EPT.
Myographic studies
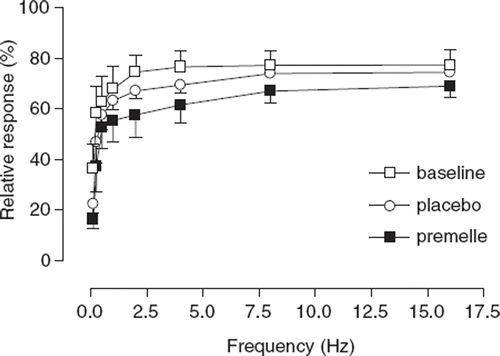
Maximal response to TNS was approximately 60-75% of the maximal NA-induced contraction and did not differ among treatments. EPT resulted in significantly decreased vascular sensitivity to cumulative TNS compared with baseline (p<0.01) and placebo (p<0.05) ().
Figure 2. Cumulative frequency-response curve for the effects of transmural nerve stimulation of subcutaneous small arteries from eight hypertensive postmenopausal women at baseline (◻) and after 6 months of treatment with placebo (◯) and Premelle® (◼). Responses are expressed as percentage of maximal response to exogenous noradrenaline. All data are expressed as means±SEM.
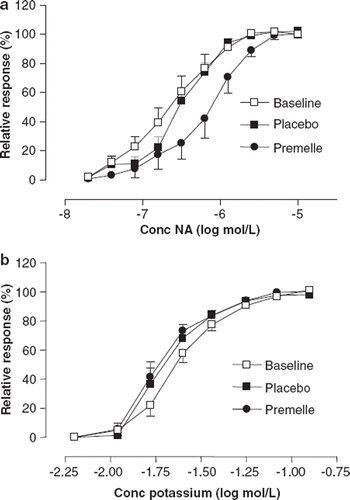
EPT decreased sensitivity to exogenously applied NA compared with baseline and placebo (p<0.05) (). Both treatment groups showed similar maximal contractile response (Emax) to NA. Further, we observed no difference in sensitivity or maximal contraction response to potassium-induced contraction ().
Figure 3. Cumulative dose-response relationships for exogenous noradrenaline (a) and potassium (b) in subcutaneous small arteries from eight hypertensive postmenopausal women at baseline (◻) and after 6 months of treatment with placebo (◼) and Premelle® (•). Responses are expressed as percent of Emax. All data are expressed as means ± SEM.
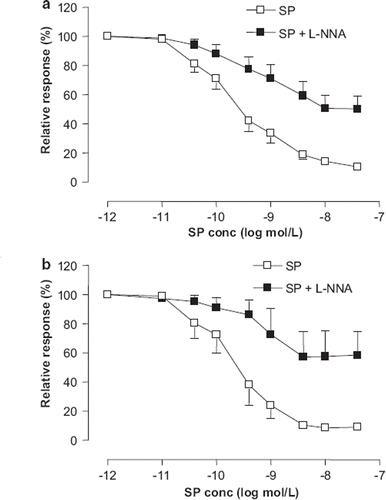
Dose-response relationships to SP remained unaffected after 6 months of EPT compared with placebo. L-NNA inhibited SP-induced relaxation (p<0.001), with no difference between treatment groups ().
Figure 4. Cumulative dose-response relations to Substance P (SP) for precontracted subcutaneous small arteries from hypertensive postmenopausal women after 6 months of treatment with placebo (a) and Premelle® (b), before (◻) and after incubation with 300 μmol/l N(ω)-nitro-L-arginine (L-NNA) (◼). Relaxation is expressed as percent of maximal response to noradrenaline. All data are expressed as means ± SEM.
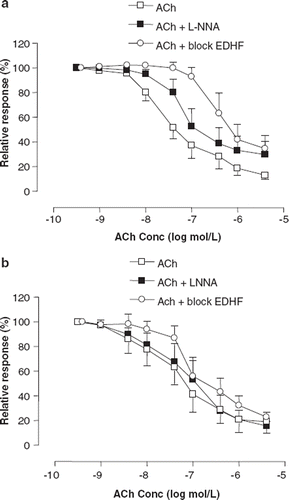
ACh (5×10-6) fully relaxed precontracted arteries after placebo treatment (87±4%) and EPT (81±9%) (). After placebo, L-NNA slightly reduced relaxation (p<0.01), with a maximal relaxation of 70±9% (), while L-NNA did not affect the dose-response relationship to ACh after EPT (). Compared with ACh+L-NNA, apamin and charybdotoxin (p<0.01) resulted in increased suppression of ACh-induced relaxation in the placebo group (). After EPT, apamin and charybdotoxin reduced relaxation slightly (p<0.05), with a maximal ACh-induced relaxation of 77±4%, even though relaxation was greater compared with placebo (p<0.01) ().
Figure 5. Cumulative dose-response relations to acetylcholine (ACh) for precontracted subcutaneous small arteries from hypertensive postmenopausal women after 6 months of treatment with placebo (a) and Premelle® (b), before (◻) and after incubation with either 300 μmol/l N(ω)-nitro-L-arginine (L-NNA) (◼) or 300 μmol/l L-NNA, charybdotoxin 0.1 μmol/l and apamin 0,7 μmol/l (block EDHF) (◯). Relaxation is expressed as percent of maximal response to noradrenaline. All data are expressed as means ± SEM.
Discussion
The present hypothesis-generating study demonstrated an attenuated adrenergic reactivity in subcutaneous arteries from hypertensive postmenopausal women during medium-term EPT. Additionally, EPT modified endothelial function, thus reinforcing the response to ACh, while the NO-dependent relaxation to SP remained unaffected. We observed only limited changes in 24-h BP, in the presence of different antihypertensive drugs. By recording hemodynamic reactivity during mental stress or by HRV measurements, we found no significant effect on the sympatho-vagal system by 6 months of EPT. The women included were treated with one to several different antihypertensive drugs. Since these drugs might interfere with the results, each individual acted as her own control and were opposed to both placebo and active treatment regimens.
Previous studies have shown an increased adrenergic vasoconstriction of mesenteric arteries of spontaneously hypertensive male rats (SHR) compared with normotensive controls (Citation19), a difference that was not present in the intact or 17β-estradiol treated female counterparts (Citation15). However, the arteries from ovariectomized female SHR showed increased response to adrenergic nerve stimulation (TNS), suggesting that estrogen deficiency involves an increased endogenous adrenergic response in hypertensive subjects (Citation13). Concordant with these experiments on animals, our study shows that EPT reduces vascular adrenergic response in hypertensive post-menopausal women, with an attenuated response to TNS in the small subcutaneous resistance arteries of such subjects. We also observed concomitantly reduced sensitivity to exogenously applied NA following EPT. The vascular smooth muscle contraction per se was not affected, as the dose–response curves of KCl remained unchanged. The hypothesis that estrogen specifically affects adrenoceptor-mediated vasoconstriction is supported by Sudhir et al. (Citation20) who demonstrated a reduced forearm asoconstrictor response to norepineprine, with no effect on angiotensin II-induced vasoconstriction, in perimenopausal women who underwent estrogen treatment for 8 weeks. Our results endorse the theory that ovarian hormones affect the adrenergic alpha receptor postjunctionally by decreasing the number of receptors (Citation21) and disapprove that ERT enhances beta-adrenoceptor-mediated vasorelaxation (Citation22), as propranolol was present during the experiment.
Endothelial function is impaired in hypertensive individuals (Citation23) because of impaired NO-production and decreased EDHF-mediated relaxation (Citation24). In the present study, the cumulative dose–response relationships show that the highest doses of SP and ACh fully relaxed subcutaneous vessels from hypertensive post-menopausal women, which might imply endothelial improvement by ongoing antihypertensive treatment.
Incubation of vessels with 17β-estradiol ex vivo, as well as acute 17β-estradiol or ET to postmenopausal women, have previously been shown to improve endothelial function (Citation25,Citation26).
EPT did not affect SP-induced relaxation, mediated mainly by NO, which is in accordance with previous results on 17β-estradiol-treated normotensive postmenopausal women (Citation27).
We have previously shown that ACh does not primarily release NO in subcutaneous arteries from normotensive postmenopausal women (Citation27). In hypertensive postmenopausal women, on the other hand, a small release of NO seems to contribute to the ACh-induced relaxation in the placebo situation. However, after 6 months of EPT for these women, ACh did not release any NO while the maximal relaxation capacity was unaffected. These findings suggest that estrogen might alter the ACh-induced endothelial release of NO in subcutaneous vessels. In agreement with these data, Nawate et al. showed that ovariectomy reduced EDHF and increased ACh release of NO in mesenteric arteries of female rats, while relaxation capacity per se remained unchanged compared with controls (Citation28). Furthermore, concordant with our previous results using 17β-estradiol (Citation27), the non-NO, non-EDHF-dependent part of ACh-induced relaxation improved following EPT.
The mechanisms behind these findings are beyond the scope of this study, but we found no evidence that EPT influences the smooth muscle membrane potential, while the dose–response relationship to potassium was unaffected by EPT. Others postulated previously that decreased acetylcholinesterase activity (Citation29) and/or increased numbers of muscarinic receptors (Citation30) account for estrogen's effect on ACh response in animal models.
We did not consider the possibility of an increased release of prostanoids, but such release seems unlikely. Indeed, previous studies have not shown a concomitant vascular release of prostacyclin by ACh following estrogen treatment, neither in postmenopausal women nor in animals (Citation27,Citation30).
Previous investigations regarding replacement therapy with different kinds of progestogens in combination with 17-β estradiol have revealed clinically significant blood pressure reduction, already after a short treatment period, both in combination with regular antihypertensive therapy and when hormonal treatment was administered alone (Citation31,Citation32). We did observe a small interaction following EPT in the 24-h ambulatory SBP curve compared with baseline and placebo treatment, and the curve was more blunted during the stressful morning hours, possibly because of reduced vascular adrenergic sensitivity to NA. Other explanations might involve a lower sympathetic drive, shown as reduced total spillover of norepinephrine (Citation20), or altered sympathovagal balance (Citation14). Because the study design aimed towards normalized BP and several antihypertensive drugs were administered parallel to hormonal treatment, it was unsurprising that we detected no difference in office BP between treatment groups.
The present study does not confirm increased vagal activity or decreased sympathetic output of EPT in hypertensive postmenopausal women, as we observed no effect on HRV measurements or hemo-dynamic responses to stress. Some earlier studies showed that estrogen affects HRV (Citation14), suggesting that ET plays a cardioprotective role by reducing sympathetic and favoring parasympathetic control of the cardiovascular system (Citation33). The discrepancy in our results might result from the counteractive effect of progesterone (Citation34,Citation35). Compared with unopposed estrogen and transdermal 17β-estradiol, orally administered estrogen and conjugated equine estrogen plus medroxyprogesterone cause fewer effects on BP and hemodynamic responses to stress (Citation8,Citation36).
This hypothesis-generating study has some limitations. All subjects were hypertensive and in need of antihypertensive therapy. This therapy might interfere with BP as well as the central adrenergic mechanisms investigated. Since the study was conducted during 1 year, it was not ethical to withdraw the antihypertensive therapy. The sample size in the vascular part of the study was small. Not all of the 20 women included in the study agreed to the biopsy part and furthermore, as three biopsies per subject were needed for evaluation, only eight women completed the myographic part. Moreover, spectral analysis was used for measuring central adrenergic reactivity, and 20 individuals might be a too limited sample for conclusive results with this method. This may have led to an underestimation of the effects of EPT on central control level. Finally, as mentioned previously, progesterone might have a different influence on vascular regulation compared with estrogen, which may result in a blunted estrogen-mediated effect.
In summary, oral combined medium-term EPT attenuates peripheral sensitivity to the stress hormone NA in anti-hypertensive treated postmenopausal women, likely contributing to improved peripheral blood flow to local tissues despite unchanged BP. The present study does not indicate an effect of EPT on the centrally mediated sympathovagal balance in hypertensive postmenopausal women. Conjugated estrogen and medroxyprogesterone, i.e. Premelle®, might act postjunctionally at the adrenergic vascular receptor level with reduced sensitivity but unaffected maximal response to adrenergic stimulation. Although relaxation capacity per se was equivalent after EPT, altered vascular relaxation to ACh suggests modulated endothelial function.
Acknowledgements
Sponsorship: Tablets with active drug and placebo, kindly provided by Organon AB, Sweden.
Part of this work was presented orally at the 14th European Meeting on Hypertension, Paris 2004 and published as an abstract in J Hypertension 22 (Suppl 2):5B.01
We thank Irene Andersson and research nurse Lill Alnäs for skilful technical assistance. We also acknowledge Organon AB for kindly providing us with active substances and placebo correlate.
Declaration of interest: The authors have no conflict of interest to declare, and no competing financial interests exist.
References
- Staessen J, Bulpitt CJ, Fagard R, Lijnen P, Amery A. The influence of menopause on blood pressure. J Hum Hypertens. 1989;3:427–433.
- Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA. 1996;275:1571–6.
- Nelson HD, Humphrey HH, Nygren P, Teutsch SM, Allan JD. Postmenopausal replacement therapy: Scientific review. JAMA. 2002;288:872–881.
- Amigoni S, Morelli P, Parazzini F, Chatenoud L. Determinants of elevated blood pressure in women around meno-pause: Results from a cross-sectional study in Italy. Maturitas. 2000;34:25–32.
- The Writing Group for the PEPI Trial. Effects of estrogen or estrogen/progestin regimens on heart disease risk factors in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1995;273:199–208.
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, . Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333.
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613.
- Mueck AO, Seeger H. Effect of hormone therapy on BP in normotensive and hypertensive postmenopausal women. Maturitas. 2004;49:189–203.
- Manuck SB, Adams MR, McCaffery JM, Kaplan JR. Behaviorally elicited heart rate reactivity and atherosclerosis in ovariectomized cynomolgus monkeys (Macaca fascicularis). Arterioscler Thromb Vasc Biol. 1997;17:1774–1779.
- Bedi M, Varshney VP, Babbar R. Role of cardiovascular reactivity to mental stress in predicting future hypertension. Clin Exp Hypertens. 2000;22:1–22.
- Bairey Merz CN, Kop W, Krantz DS, Helmers KF, Berman DS, Rozanski A. Cardiovascular stress response and coronary artery disease: Evidence of an adverse postmenopausal effect in women. Am Heart J. 1998;135:881–887.
- Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167:1831–1836.
- He XR, Wang W, Crofton JT, Share L. Effects of 17beta-estradiol on sympathetic activity and pressor response to phenylephrine in ovariectomized rats. Am J Physiol. 1998;275:R1202–R1208.
- Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. 2003;285:H2188–H2193.
- Brandin L, Bergstrom G, Manhem K, Gustafsson H. Oestrogen modulates vascular adrenergic reactivity of the spontaneously hypertensive rat. J Hypertens. 2003;21:1695–1702.
- Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26.
- Chen G, Cheung DW. Effect of K(+)-channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. Am J Physiol. 1997;272:H2306–H2312.
- Kuo TB, Linn T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol. 1999;277:H2233–H2239.
- Chang HR, Lee RP, Wu CY, Chen HI. Nitric oxide in mesenteric vascular reactivity: A comparison between rats with normotension and hypertension. Clin Exp Pharmacol Physiol. 2002;29:275–280.
- Sudhir K, Elser MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30:1538–1543.
- Ford SP, Reynolds LP, Farley DB, Bhatnagar RK, Van Orden DE. Interaction of ovarian steroids and periarterial alpha 1-adrenergic receptors in altering uterine blood flow during the estrous cycle of gilts. Am J Obstet Gynecol. 1984;150:480–484.
- Ferrer M, Meyer M, Osol G. Estrogen replacement increases beta-adrenoceptor-mediated relaxation of rat mesenteric arteries. J Vasc Res. 1996;33:124–133.
- Higashi Y, Sanada M, Sasaki S, Nakagawa K, Goto C, Matsuura H, Ohama K, Chayama K, Oshima T. Effect of estrogen replacement therapy on endothelial function in peripheral resistance arteries in normotensive and hypertensive postmenopausal women. Hypertension. 2001;37:651–657.
- Sunano S, Watanabe H, Tanaka S, Sekiguchi F, Shimamura K. Endothelium-derived relaxing, contracting and hyperpolarizing factors of mesenteric arteries of hypertensive and normotensive rats. Br J Pharmacol. 1999;126:709–716.
- Lima SM, Aldrighi JM, Consolim-Colombo FM, Mansur Ade P, Rubira MC, Krieger EM. Acute administration of 17beta-estradiol improves endothelium-dependent vasodilation in postmenopausal women. Maturitas. 2005;50:266–274.
- Kublickiene K, Svedas E, Landgren BM, Crisby M, Nahar N, Nisell H, Poston L. Small artery endothelial dysfunction in postmenopausal women: In vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metab. 2005;90:6113–6122.
- Manhem K, Brandin L, Ghanoum B, Rosengren A, Gustafsson H. Acute effects of transdermal estrogen on hemodynamic and vascular reactivity in elderly postmenopausal healthy women. J Hypertens. 2003;21:387–394.
- Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Br J Pharmacol. 2005;144:178–189.
- Cheng DY, Feng CJ, Kadowitz PJ, Gruetter CA. Effects of 17 beta-estradiol on endothelium-dependent relaxation induced by acetylcholine in female rat aorta. Life Sci. 1994;55:PL187–PL191.
- Gisclard V, Miller VM, Vanhoutte PM. Effect of 17 beta-estradiol on endothelium-dependent responses in the rabbit. J Pharmacol Exp Ther. 1988;244:19–22.
- Preston RA, Norris PM, Alonso AB, Ni P, Hanes V, Karara AH. Randomized, placebo-controlled trial of the effects of drospirenone-estradiol on blood pressure and potassium balance in hypertensive postmenopausal women receiving hydro-chlorothiazide. Menopause. 2007;14:408–414.
- White WB, Pitt B, Preston RA, Hanes V. Antihypertensive effects of drospirenone with 17β-estradiol, a novel hormone treatment in postmenopausal women with stage 1 hypertension. Circulation. 2005;112:1979–1983.
- Janszky I, Ericson M, Mittleman MA, Wamala S, Al-Khalili S, Schenk-Gustafsson K. Heart rate variability in long-term risk assessment in middle-aged women with coronary heart disease: The Stockholm Female Coronary Risk Study. J Intern Med. 2004;255:13–21.
- Niskanen L, Laitinen T, Tuppurainen M, Saarikoski S, Kroger H, Alhava E. Does postmenopausal hormone replacement therapy affect cardiac autonomic regulation in osteoporotic women? Menopause. 2002;9:52–57.
- Rosa Brito-Zurita O, Posadas-Romero C, Hermosillo AG, Zamora-Gonzalez J, Hernandez-Ono A, Cardoso-Saldana G. Estrogen effect on heart rate variability in hypertensive postmenopausal women. Maturitas. 2003;44:39–48.
- Girdler SS, Hinderliter AL, Wells EC, Sherwood A, Grewen KM, Light KC. Transdermal versus oral estrogen therapy in postmenopausal smokers: Hemodynamic and endothelial effects. Obstet Gynecol. 2004;103:169–180.