Abstract
Objective. It is unclear whether improvement of left atrial (LA) and ventricular (LV) structure results in reduction in new-onset atrial fibrillation (AF). The aim of the present study was to examine whether changes in-treatment LA diameter were related to changes in risk of new-onset AF. Methods. We followed 939 hypertensive patients with electrocardiographic LV hypertrophy randomized to atenolol or losartan-based regimens in the LIFE Study for a mean of 4.8 years with echocardiograms at enrolment and annually during treatment. Results. New-onset AF occurred in 46 patients (10.2/1000 patient-years of follow-up). At baseline, patients with new-onset AF were older, had higher systolic blood pressure and heart rate as well as higher prevalence of LA dilatation, but had similar prevalences of LV hypertrophy and mitral or aortic valve disease. In univariate Cox analysis baseline LA diameter (HR=4.67 per cm increase [95% CI 2.86–7.65], p<0.001) and LV mass index (HR=1.11 per 10 g/m2 increase [95% CI 1.02–1.22], p<0.05) both predicted new-onset AF. In multivariate analysis, increased baseline LA diameter increased the risk of new-onset AF (HR=5.16 per cm [95% CI 2.85–9.35], p<0.001) whereas reduction of in-treatment LA diameter reduced the risk (HR=0.21 per cm lower LA diameter during treatment [95% CI 0.14–0.32], p<0.001) with adjustment for in-treatment LV mass in-treatment systolic blood pressure, age and Framingham risk score. Conclusion. LA diameter at baseline and during antihypertensive treatment were equally strong predictors of new-onset AF independent of the level of arterial pressure, LV mass and other covariates. Prevention of AF during antihypertensive treatment may be improved by antihypertensive therapy that reduces LA size in addition to controlling blood pressure.
Introduction
Several studies in the general population and hypertensive patients have identified left atrial (LA) enlargement as a cardiovascular risk marker, in particular for atrial fibrillation (AF) and stroke (Citation1–4). We have published data from a large population of patients with essential hypertension and electrocardiographic-verified left ventricular (LV) hypertrophy from the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study (Citation5) showing a 33% risk reduction in new-onset AF in favor of losartan (Citation6). Furthermore, we found that the rate of cardiovascular morbidity and mortality, including stroke, in patients with a history of AF at baseline was reduced by losartan treatment (Citation7). Both papers suggested that changes in LA diameter might contribute to the effects of losartan on AF. In a subset of LIFE patients who underwent echocardiography, we have showed that LA diameter was reduced more by losartan than by atenolol-based therapy as well as its in-treatment changes predicted major cardiovascular events independent of the change in blood pressure during antihypertensive treatment (Citation8).
However, to our knowledge, there are no data that clarify whether reductions in LA diameter and LV mass during antihypertensive treatment predict reduced risk of new-onset AF. Thus, the aim of the present study was to examine whether changes in LA diameter and LV hypertrophy during antihypertensive treatment in the LIFE study was associated with reduced risk of new-onset AF.
Materials and methods
Patients and design
As part of the Losartan Intervention For Endpoint (LIFE) trial, over 10% of study participants enrolled in a substudy in which echocardiograms were performed at study baseline and yearly thereafter (Citation9–12). The present report uses the echocardiograms at baseline and annual clinical visits and endpoints collected during 4493 patient-years of follow-up. The main LIFE outcome (Citation5) as well as the complete study protocol with study design, organization, clinical measures, endpoint definitions, exclusion criteria, basis for choice of comparative agents, statistical considerations and baseline characteristics have been previously published (Citation5,Citation7,Citation13–15).
Eligible individuals were 960 patients with stage II–III hypertension enrolled in the LIFE Echocardiography Substudy (Citation9–12). However, 21 participants in the echocardiography substudy were ineligible for the present study because of inability to obtain baseline measurements of LA diameter. Compared with the ineligible patients, the present study population (n=939) were at enrollment younger (65.9±6.7 vs 69.5±6.3 years, p<0.05) and had lower body mass index (27.2±4.4 vs 30.1±6.2 kg/m2, p<0.05) but did not differ in gender distribution, history of AF, diabetes, coronary, cerebral or peripheral vascular disease or systolic or diastolic blood pressure (data not shown).
The LIFE Echocardiography Substudy was carried out in selected echocardiography centers in Denmark, Finland, Iceland, Norway, Sweden, UK and the USA with a geographic distribution, mean blood pressure and body mass index and prevalences of diabetes and vascular disease resembling the entire LIFE population with the exception of enrolling more men and non-white participants (Citation12). Patients gave informed consent and ethics committees in participating countries accepted this study. Prior to enrollment in the study, all patients had a screening electrocardiogram (ECG) showing LV hypertrophy by either sex-adjusted Cornell voltage–duration product ≥2440 mV×ms or Sokolow–Lyon voltage criteria >38 mV (Citation16). Further inclusion criteria included lack of myocardial infarction or stroke within 6 months, absence of current congestive heart failure, known LV ejection fraction <40%, significant aortic stenosis, or overt renal insufficiency (serum creatinine >160 µmol/l or 1.8 mg/dl).
Treatment regimens
Blinded treatment was begun with 50 mg of losartan or atenolol; respectively. During the following visits at 1, 2, 4 and 6 months and semiannually thereafter, study therapy was up-titrated by adding hydrochlorothiazide 12.5 mg, followed by 100 mg losartan or atenolol; respectively in order to reach target blood pressure ≤140/90 mmHg. Further increase in hydrochlorothiazide and drugs other than angiotensin converting enzyme inhibitor/angiotensin-II-/beta-blockers antihypertensive medication could be initiated by investigators.
Echocardiographic methods
Echocardiographic procedures for this study are previously described (Citation9–12). End-diastolic LV dimensions were used to calculate LV mass by an anatomically validated formula (r=0.90 versus necropsy LV mass) (Citation17); LV mass showed excellent inter-study reliability (Citation18). LV hypertrophy was considered present when LV mass indexed by body surface area was >116 g/m2 for men and >104 g/m2 for women (Citation19). LA diameter was measured in long-axis views from the trailing edge of the posterior aortic-anterior LA complex to avoid including the variable diameter of the connective tissue filled space between these structures erroneously in atrial diameter, as previously reported (Citation20). The LA diameter was considered enlarged when LA diameter exceeded 3.8 cm in women and 4.2 cm in men, the upper limit of the 95% confidence interval of LA diameter found in 413 apparently normal adults in another study from the Reading Center (Citation20). Aortic and mitral regurgitation were assessed by color Doppler using previously described 4-point grading systems (Citation21,Citation22).
Endpoint determination
New-onset AF was identified from annual in-study ECGs, which underwent Minnesota coding for AF at a single ECG Core Center (Citation16). Care of the patients' new-onset AF was left to the discretion of local investigators. Prevalent coronary, cerebral or peripheral vascular disease and smoking habits were reported by patients and investigators and independently source-verified. Framingham risk score (Citation23) was calculated from baseline blood pressure, total-cholesterol, high-density lipoprotein-cholesterol, smoking, diabetes and electrocardiographic LV hypertrophy.
Statistical methods
Data management and analyses were performed by investigators using SPSS software ver. 17.0 (SPSS, Inc. Chicago, IL) was used for statistical analysis. Results are mean±standard deviation (SD) or frequencies expressed as percentages. Independent correlates of LA diameter were identified by multiple linear regression or logistic regression analysis, where appropriate, using an enter procedure with assessment of colinearity. Aortic or mitral regurgitation were dichotomized as either no or discrete or ≥grade 1. Aortic valvular stenosis was dichotomized as either none or as ≥mild aortic stenosis.
All endpoints were analyzed using the intention-to-treat approach; all randomized patients with baseline LA measurements were included in their randomized treatment group, and all available follow-up data were included from randomization until study termination date.
In order to determine whether in-treatment LA diameter and LV mass were predictors of new-onset AF, hazard ratios (HR) for in-treatment time-varying LA diameter and in-treatment time-varying LV mass index were calculated using Cox models (Citation15,Citation24). Baseline systolic and diastolic blood pressure, and a treatment group indicator were standard covariates; systolic and diastolic blood pressure during treatment were additional time-varying covariates. The time-varying covariate value associated with an event time used the most recent measurement prior to the event. Note that in-treatment values of LA diameter and LV hypertrophy were used in these analyses rather than their changes from baseline because changes are highly correlated with baseline values. For example, patients with the largest baseline LA diameter had the greatest in-treatment diameter reductions but still had on average the highest in-treatment LA diameter, and thereby would still be at the highest risk of incident AF (Citation15), which would bias an analysis of changes. Further analyses of LA dilatation (>3.8 cm in women and >4.2 cm in men) are displayed using modified Kaplan–Meier curves; the modification is that the risk sets for each LA dilatation category change over time, and patients can shift among the different cohorts as their LA dilatation classification changes over the study process. Two-tailed p<0.05 was considered statistically significant.
Results
Baseline characteristics for the total LIFE Echocardiography population have been reported previously (Citation9,Citation12). New-onset AF occurred in 46 (10.2/1000 patient-years of follow-up) patients of 939 participants in the LIFE Echocardiography study that had baseline measurements necessary for inclusion in this analysis. Patients with new-onset AF were older, had higher systolic blood pressure, heart rate and Framingham risk score as well as more prevalent LA dilatation. Gender, diastolic blood pressure, LV hypertrophy as well as mitral or aortic valve disease did not differ between groups (). Although the rate of new-onset AF was 25% less in losartan- than atenolol-treated patients, this result did not attain statistical significance in the present subset of the LIFE population, most likely because of a type II error.
Table I. Demographic and clinical characteristic in relation to development of new-onset atrial fibrillation.
Baseline LA size strongly predicted new-onset AF. Compared with the first quartile of baseline LA size there was a stepwise increase in the rates of new-onset AF (second quartile: HR=8.64 [95% CI 1.09–68.2], p=0.041, third quartile: HR=12.24 [95% CI 1.55–96.8], p=0.018 and fourth quartile: HR=23.9 [95% CI 3.24–176.2] p=0.002), with p<0.001 for trend. In univariate Cox regression analyses, baseline LA diameter, interventricular septal thickness and LV mass predicted new-onset AF (), whereas LV internal dimension, circumferential end-systolic stress and baseline mitral regurgitation did not. When both LA diameter and LV mass were entered in a multivariate Cox analysis including, only baseline LA diameter remained a predictor (HR=5.16 [95% CI 2.85–9.35], p<0.001) of new-onset AF.
Table II. Univariate Cox proportional hazard models for baseline left atrial and ventricular structure for the prediction of new-onset atrial fibrillation during antihypertensive treatment.
Mean changes in LA diameter were related to baseline LA diameter with progressively greater reduction in LA diameter during the 1st and 2nd year of study treatment from the lowest to the highest quartile of baseline LA diameter at enrollment (0.23±0.52 cm the first baseline LA quartile, −0.02±0.47 cm in the second, −0.15±0.46 cm in the third and −0.31±0.56 cm in the fourth quartile, p<0.001 for trend).
Cox regression analyses using time-varying LA diameter, controlling for in-treatment LV mass, in-treatment systolic blood and baseline Framingham risk score, randomized study treatment and age (), revealed that time-varying LA diameter (HR=0.21 per 1 cm reduction in LA diameter during treatment [95% CI 0.14–0.32], p<0.001) was the sole independent predictor of new-onset AF (). We have previously reported that losartan reduced LA diameter more than atenolol in LIFE echo substudy participants (0.15±0.51 vs 0.01±0.58 cm, p<0.001) from baseline to the last available measurement (Citation8). In a similar model with similar adjustments as shown in , a 0.14-cm reduction in LA would be associated with a 21% reduction in new-onset AF (HR=0.79 [95% CI 0.75–0.84], p<0.001). Also noted in , the HR of losartan was reduced to only 6% (HR=0.94 [0.54–1.72], p=0.844) when taking time-varying LA size into account. In order to estimate the attributable effect of losartan on the LA size a separate model excluding time-varying LA size showed that losartan reduced new-onset AF by 28% (HR=0.72 [0.40–1.30], p=0.278) when adjusting for time-varying systolic blood pressure, time-varying LV mass by echocardiography as well as Framingham risk score (full model not shown).
Figure 1. Rates of new-onset atrial fibrillation stratified by in-treatment presence or absence of left atrial dilatation using partition values 4.2 cm for men and 3.8 cm for women (patients are assigned to each group on the basis of their left atrial size measured by baseline, semi-annual or annual echocardiogram and may variably be included in one curve or another during follow-up, illustrating time-varying categorical left atrial size as a predictor of new-onset atrial fibrillation).
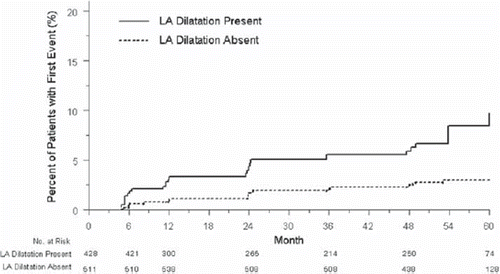
Table III. Multivariate Cox proportional hazard models of time-varying left atrial and ventricular structure for the prediction of new-onset atrial fibrillation.
Discussion
The primary objective of this study was to evaluate the relation of measures of LA and LV structure to the risk of new-onset AF during antihypertensive treatment. Our study confirms not only that LA enlargement at baseline an important predictor of new-onset AF (Citation1–4) but provides the first evidence that LA diameter reduction during antihypertensive treatment is an even stronger correlate of lower rate of new-onset AF (risk reduction 79% per cm lower in-treatment LA diameter) even when taking in-treatment LV mass index and systolic blood pressure into account. We have in other analyses from the LIFE Echocardiography study shown that change in in-treatment LA diameter was an important predictor of cardiovascular morbidity and mortality, especially fatal and non-fatal stroke.(Citation8) Although, in the current study the rate of new-onset AF was non-significant 25% less in losartan- than atenolol-treated patients, this result is most likely because of a type II error as the current population is a representative sample of the total LIFE population where a 33% reduction in new-onset AF favor of losartan did attain strong statistical significance(Citation6).
In addition, we have found that new-onset AF is strongly associated with increased cardiac morbidity and mortality (Citation6). Patients in the LIFE-study with new-onset-AF had a more than threefold higher risk of fatal and non-fatal stroke compared with patients that remained in sinus rhythm (Citation6). The present study also extends our previous documentation indicating reduction in electrocardiographic LV hypertrophy is associated with a lower rate of new-onset AF (Citation25). The time-dependent analysis, controlling for changes in LV mass and systolic blood pressure, indicates that the effect of antihypertensive treatment on LA diameter independently influences the likelihood of developing AF. This may suggest that normalization of LA diameter, at least in hypertensive patients with ECG LV hypertrophy, may represent a new goal for antihypertensive treatment. This possibility will require confirmation in other populations before it can be accepted as a standard of practice.
We have previously reported that losartan reduced LA diameter more than atenolol in LIFE echo substudy participants (0.15±0.51 vs 0.01±0.58 cm, p<0.001) from baseline to the last available measurement (Citation8). The current study puts into perspective the mechanism by which losartan was associated with a 33% risk reduction for new-onset AF in LIFE patients (n=8,851) without reported AF at baseline (Citation6) and a 45% risk reduction for fatal and non-fatal stroke in those who had a history of AF (Citation7). A reduction of LA size by 0.14 cm seen in losartan- compared with atenolol-treated would explain a 21% reduction in incident AF or roughly 60% of the reduction of new-onset AF by losartan vs atenolol-based therapy in the LIFE study. In addition, a reasonable interpretation would be that an appreciable part of the benefit of losartan treatment with regard to new-onset AF prevention is mediated by losartan's beneficial effect on LA size that is independent of losartan's effects on blood pressure and LV mass. We believe that this suggests that approximately 80% of the reduction in new-onset AF by losartan seen in LIFE is attributable to losartan's beneficial effect on the LA size.
Thus, our study expands knowledge regarding prevention of AF in hypertensive heart disease by identifying greater reduction in of LA dilatation with losartan-based therapy as one potential mechanism by which losartan may have reduced the rates of associated complications compared with those on atenolol-therapy. Additional possible mechanisms of losartan's benefit that was not evaluated in the LIFE study, include reduction in atrial fibrosis, improvement of atrial electrophysiological properties by prevented the promotion of AF by reducing atrial structural remodeling (Citation26). Furthermore, a recent study suggests that renin–angiotensin system polymorphisms are associated with non-familial AF (Citation27).
Potential limitations of the study include the use of LA diameter measured in long axis view as the index of LA size. Several studies support LA volumes as more accurate than LA dimension, but have also shown that the predictive values of the widely used measurement for a variety of cardiovascular endpoints is only moderately weaker than that of LA volume measurement. All patients had ECG LV hypertrophy and hypertension and were thus at high cardiovascular risk. Our reliance on annual ECGs read at a core laboratory undoubtedly missed some instances of paroxysmal AF but provides the advantage of systematic verification of this study's endpoint. In addition, annual ECGs for detecting AF may be more sensitive for detecting persistent/permanent (sustained) AF than paroxysmal AF. However, enlargement of the LA is by many regarded as a mechanism especially promoting sustained AF in contrast to paroxysmal AF. Although the analysis of AF was not pre-specified in the 1995 LIFE study analysis plan, evaluation of treatment effects in the subgroup of patients with baseline and new-onset AF were planned before study termination (September 2001) and unblinding.
In conclusion, our findings have potential important clinical implications. The increased cardiovascular risk associated AF, and in particular stroke risk associated development of new-onset AF in hypertensive patients undergoing antihypertensive treatment, make prevention of new AF a high clinical priority to reduce cardiovascular morbidity and mortality. These results together with our previous findings suggest that antihypertensive therapies that effectively reduce blood pressure and also independently reduce LA size may provide reduced risk of new-onset AF and could also reduce subsequent stroke and other cardiovascular morbidity and mortality (Citation6–8). Further study will be necessary to determine whether reduction in LA size is will be become a valid target for therapeutic intervention in hypertensive patients to prevent development of AF and other adverse cardiovascular outcomes.
Acknowledgements
The work was supported in part by a grant from editor and Mrs. Kaarsen's foundation, Copenhagen, Denmark and Merck & Co Inc, West Point, PA. KW received grant support and honoraria from Merck & Co Inc. EG received grant support and honoraria from Merck & Co Inc. GPA has no disclosures. KB received grant support from Merck & Co Inc. B.D. serves on the speaker bureau, received honoraria from Merck & Co Inc and is a consultant to Merck & Co Inc. MSN received grant support and honoraria from Merck & Co Inc. MHO received grant support and honoraria from Merck & Co Inc. PMO received grant support from Merck & Co Inc. VP received grant support from Merck & Co Inc. JER received grant support and honoraria from Merck & Co Inc. RBD received grant support and honoraria from Merck & Co Inc. and serves on an advisory board for Merck & Co Inc.
Declaration of interest: The sponsor provided the study authors with free access to all the data; the authors were free to interpret data and write the article. The sponsor agreed to support the performance of the study at which time it was agreed that the findings would be published by the investigators regardless of the results. The decision to publish the article, the choice of analyses to include, and the drafting of the manuscript were wholly controlled by KW and coauthors.
References
- Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841.
- Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, . Left atrial volume: Important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475.
- Verdecchia P, Reboldi G, Gattobigio R, Bentivoglio M, Borgioni C, Angeli F, . Atrial fibrillation in hypertension: Predictors and outcome. Hypertension. 2003;41: 218–223.
- Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, . Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026–2033.
- Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, Faire U, . Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. Lancet. 2002;359:995–1003.
- Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, . Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: The LIFE study. J Am Coll Cardiol. 2005;45: 712–719.
- Wachtell K, Hornestam B, Lehto M, Slotwiner DJ, Gerdts E, Olsen MH, . Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: The LIFE study. J Am Coll Cardiol. 2005;45:705–711.
- Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, . Left atrial size and risk of major cardiovascular events during antihypertensive treatment: Losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49:311–316.
- Wachtell K, Bella JN, Liebson PR, Gerdts E, Dahlöf B, Aalto T, . Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population: The LIFE study. Hypertension. 2000;35:6–12.
- Wachtell K, Rokkedal J, Bella JN, Aalto T, Dahlöf B, Smith G, . Effect of electrocardiographic left ventricular hypertrophy on left ventricular systolic function in systemic hypertension (The LIFE Study). Am J Cardiol. 2001;87:54–60.
- Wachtell K, Smith G, Gerdts E, Dahlöf B, Nieminen MS, Papademetriou V, . Left ventricular filling patterns in patients with systemic hypertension and left ventricular hypertrophy (the LIFE study). Am J Cardiol. 2000;85: 466–472.
- Devereux RB, Bella JN, Boman K, Gerdts E, Nieminen MS, Rokkedal J, . Echocardiographic left ventricular geometry in hypertensive patients with electrocardiographic left ventricular hypertrophy: The LIFE Study. Blood Press. 2001; 10:74–82.
- Dahlöf B, Devereux RB, Julius S, Kjeldsen SE, de Faire U, Fyhrquist F, . Characteristics of 9194 patients with left ventricular hypertrophy: The LIFE study. Hypertension. 1998;32:989–997.
- Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Dahlöf B. Baseline characteristics in relation to electrocardiographic left ventricular hypertrophy in hypertensive patients: The Losartan intervention for endpoint reduction (LIFE) in hypertension study. The Life Study Investigators. Hypertension. 2000;36:766–773.
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Dahlöf B, Devereux RB, de Faire U, Fyhrquist F, Hedner T, Ibsen H, . The Losartan Intervention For Endpoint reduction (LIFE) in Hypertension Study. Am J Hypertens. 1997;10:705–713.
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, . Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Palmieri V, Dahlöf B, DeQuattro V, Sharpe N, Bella JN, de Simone G, . Reliability of echocardiographic assessment of left ventricular structure and function: The PRESERVE study. Prospective Randomized Study Evaluating Regression of Ventricular Enlargement. J Am Coll Cardiol. 1999;34: 1625–1632.
- Devereux RB, Dahlöf B, Levy D, Pfeffer MA. Comparison of enalapril versus nifedipine to decrease left ventricular hypertrophy in systemic hypertension (the PRESERVE trial). Am J Cardiol. 1996;78:61–65.
- Ilercil A, O'Grady MJ, Roman MJ, Paranicas M, Lee ET, Welty TK, . Reference values for echocardiographic measurements in urban and rural populations of differing ethnicity: The Strong Heart Study. J Am Soc Echocardiogr. 2001;14:601–611.
- Lebowitz NE, Bella JN, Roman MJ, Liu JE, Fishman DP, Paranicas M, . Prevalence and correlates of aortic regurgitation in American Indians: The Strong Heart Study. J Am Coll Cardiol. 2000;36:461–467.
- Jones EC, Devereux RB, Roman MJ, Liu JE, Fishman D, Lee ET, . Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol. 2001;87:298–304.
- Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362.
- Chen C, Wang HW, Snapinn SM. Proportion of treatment effect (PTE) explained by a surrogate marker. Stat Med. 2003;22:3449–3459.
- Okin PM, Wachtell K, Devereux RB, Harris KE, Jern S, Kjeldsen SE, . Regression of electrocardiographic left ventricular hypertrophy and decreased incidence of new-onset atrial fibrillation in patients with hypertension. JAMA. 2006;296:1242–1248.
- Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–2204.
- Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, . Renin–angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–1646.