Abstract
Background. Some patients with aortic stenosis develop asymmetric septal hypertrophy (ASH) that may influence the surgical approach and is associated with higher perioperative morbidity. The aim of this analysis was to characterize further this subtype of aortic stenosis patients. Methods. Baseline data in 1719 patients with asymptomatic aortic stenosis, participating in the Simvastatin Ezetimibe in Aortic Stenosis study evaluating the effect of combined treatment with simvastatin and ezetimibe on progression of aortic stenosis was used. The study population was divided according to presence of ASH (interventricular septal/posterior wall thickness ratio >1.5). Left ventricular (LV) hypertrophy was considered present if LV mass index ≥104 g/m² in women and ≥116 g/m2 in men. Results. ASH was present in 22% of patients and associated with higher LV mass index, total peripheral resistance and peak transaortic velocity and concomitant hypertension (all p<0.05). Thirty-four percent of patients with ASH had combined ASH and LV hypertrophy (asymmetric LV hypertrophy). These patients had higher systolic blood pressure, lower LV ejection fraction and larger left atrial diameter than patients with ASH only. In logistic regression analyses, hypertension was the most important predictor both for ASH (odds ratio, OR 1.38 [1.05–1.82]) and for asymmetric LV hypertrophy (OR 2.99 [1.71–5.25]), both p<0.05) independent of other covariates including severity of aortic stenosis. Conclusion. Hypertension is the main clinic characteristic of ASH and asymmetric LV hypertrophy in patients with asymptomatic aortic stenosis independent of severity of aortic stenosis.
Introduction
Although asymmetric septal hypertrophy (ASH) mostly has been associated with hypertrophic cardiomyopathy (Citation1), ASH has also long been recognized as an early and frequent structural adaptation in hypertension (Citation2–4). In particular, ASH has been linked to clinical characteristics in hypertension associated with increased cardiovascular risk (Citation2).
There has been less focus on ASH in patients with aortic stenosis although degenerative aortic stenosis is found in 2.5% of patients older than 65 years and is the most frequent cause of valve surgery in developed countries (Citation5). Moreover, presence of ASH in patients with aortic stenosis may influence the surgical approach and has also been associated with higher perioperative morbidity (Citation6,Citation7). Thus, the aim of the present analysis was to identify covariates of ASH in patients with asymptomatic aortic stenosis participating in the Simvastatin Ezetimibe in Aortic Stenosis (SEAS) study.
Materials and Methods
Patient population
The present analysis was prospectively planned within the SEAS study, which enrolled 1873 patients aged 45–85 years with asymptomatic aortic valve stenosis (Doppler-measured peak transaortic jet velocity ≥2.5 m/s and ≤4.0 m/s) to a 4-year, placebo-controlled treatment with combined simvastatin 40 mg and ezetimibe 10 mg daily to evaluate the effect on progression of aortic stenosis and associated cardiovascular events including cardiovascular death, aortic valve replacement, heart failure as a result of progression of aortic stenosis, non-fatal myocardial infarction, coronary artery disease, hospitalized unstable angina and non-hemorrhagic stroke. Study organization, design, patient recruitment and outcome have previously been published (Citation8,Citation9). Patients with other significant valvular diseases, rheumatic valvular disease, prosthetic valves, previous stroke, a history of coronary artery disease or diabetes were not included in the SEAS study. All patients gave written informed consent and ethical committees in all participating countries approved the study. The present study population consisted of the 1719 patients (91.7% of all included patients) in whom LV dimensions were measurable on the baseline study echocardiogram. Compared with the present study population, excluded patients had higher body mass index (28.6 vs 26.8 kg/m2) and diastolic blood pressure (86 vs 82 mmHg) and included fewer women (21 vs 39%, all p<0.05), while age and prevalence of hypertension did not differ. Hypertension was defined as history of hypertension or baseline clinic systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.
Echocardiographic protocol
A total of 173 centers in Denmark, Finland, Germany, Ireland, Norway, Sweden and UK with established expertise in quantitative echocardiography participated in the study. The standardized echocardiography protocol and performance in the SEAS study have been previously published (Citation8,Citation9). All images were recorded on S-VHS, CD or MO disks and sent for central, blinded interpretation at the SEAS echocardiography core laboratory at Haukeland University Hospital in Bergen, Norway. All examinations were initially read by a junior member of the staff and later proof-read by a senior investigator; 93% of final readings were performed by one highly experienced reader (EG). All reading was performed using off-line digital workstations with Image Arena® (TomTec Imaging Systems GmbH, Unterschleissheim, Germany) software.
Echocardiographic measurements
LV dimensions and wall thicknesses were measured according to the American Society of Echocardiography recommendations (Citation10). LV volumes and ejection fraction were estimated by biplane Simpson’s method and used for calculation of stroke volume and cardiac output (Citation10). Pulse pressure/stroke volume index was calculated as an indirect measure of systemic arterial stiffness. Total peripheral resistance was calculated from mean arterial pressure and cardiac output. LV mass was calculated using an autopsy validated formula (Citation11). LV hypertrophy was considered present using prognostically validated cut-offs for LV mass index (LV mass/body surface area) ≥104 g/m² in women and ≥116 g/m² in men (Citation12). ASH was defined as interventricular septal/posterior wall thickness ratio >1.5. Asymmetric LV hypertrophy was considered present if LV hypertrophy and ASH coincided.
Aortic annular diameter was measured by an inner-edge to inner-edge method (Citation10). Aortic valve area was calculated using the continuity equation with Doppler time–velocity integrals and indexed for body surface area. Transvalvular pressure drop was calculated by Bernoulli’s equation. Heart rate was measured from the echocardiogram and blood pressures measured at the end of the echocardiographic examination in supine position were used for calculation of hemodynamic variables.
Statistical analysis
Data management and statistical analysis were performed using SPSS software version 15.0 (SPSS, Chicago, IL). Data are expressed as mean±SD for continuous variables and as percentages for categorical variables. Differences between groups were assessed by independent sample t-tests and by χ2 tests, as appropriate. Univariate correlations were assessed by Person’s correlation coefficients. Independent correlates of ASH and asymmetric LV hypertrophy were identified by multiple logistic regression analyses and are reported as odds ratio (OR) and 95% confidence intervals (CI). Two-tailed p-value of <0.05 was considered statistically significant both in univariate and multivariate analyses.
Results
Asymmetric septal hypertrophy
ASH was found in 22% (n=381) of the total study population (n=1719). Compared with patients without ASH, patients with ASH included more patients with hypertension (p<0.01) (, ). Patients with ASH also had higher LV mass index, total peripheral resistance, pulse pressure/stroke volume index and more severe aortic stenosis (all p<0.05) ().
Table I. Clinical findings in groups of patients with or without asymmetric septal hypertrophy (ASH).
Figure 1. Prevalences of asymmetric septal hypertrophy (ASH) and asymmetric left ventricular hypertrophy (asymmetric LVH) in nor-motensive (left) and hypertensive patients (right).
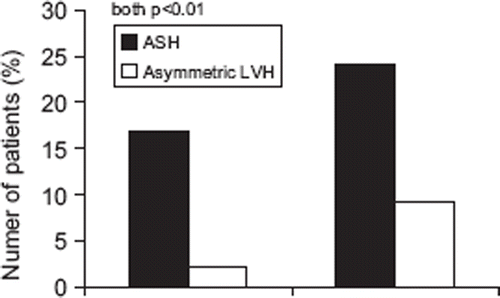
Table II. Echocardiographic findings in groups of patients with or with out asymmetric septal hypertrophy (ASH).
In univariate analysis, higher septal/posterior wall thickness ratio was weakly but significantly associated with higher body mass index (r=0.07), LV mass index (r=0.12), total peripheral resistance (r=0.09), pulse pressure/stroke volume index (r=0.10) (all p<0.01) and more severe aortic valve stenosis (higher transvalvular peak gradient, r=0.05 and peak aortic jet velocity, r=0.05, both p<0.05). From univariate associations, a multiple logistic regression model was constructed to identify independent covariates of ASH demonstrating that hypertension was the most important covariate of ASH (OR 1.38 [1.05–1.82], p=0.023), followed by LV mass index and total peripheral resistance (). Weight, body surface area, body mass index, age and gender were not identified as independent covariates of ASH.
Table III. Factors associated with asymmetric septal hypertrophy (ASH) in the total study population; multivariate logistic regression analysis.
Asymmetric LV hypertrophy
Among the 381 patients with ASH, 34% (n=130) also had LV hypertrophy. In the total study population, combined ASH and LV hypertrophy, called asymmetric LV hypertrophy was significantly more common in hypertensive patients (). Compared with patients with ASH without LV hypertrophy, the subgroup of patients with asymmetric LV hypertrophy, had higher blood pressure and prevalence of hypertension and lower heart rate (), while prevalence of drug treated hypertension (51 vs 59%) or use of beta-blockers (41 vs 34%, both p=ns) did not differ between these groups. Patients with asymmetric LV hypertrophy also had larger left atrial diameter, lower LV ejection fraction and total peripheral resistance and more severe aortic valve stenosis than patients with ASH only (). In multiple logistic regression, hypertension (OR 2.99 [1.71–5.25], p<0.001) was the most important covariate of asymmetric LV hypertrophy ().
Table IV. Clinical findings in patients with asymmetric septal hypertrophy divided into groups with (asymmetric LVH) or without (ASH) concomitant LVH.
Table V. Echocardiographic findings in patients with asymmetric septal hypertrophy with (asymmetric LVH) or without (ASH) concomitant LVH.
Table VI. Factors associated with asymmetric LV hypertrophy (LVH) in the total study population; multivariate binary logistic regression analysis.
Discussion
While ASH predominantly has been associated with hypertrophic cardiomyopathy in the medical literature, ASH is also found in subpopulations of hypertensive as well as aortic stenosis patients (Citation1–6,Citation13–15). Presence of ASH in patients with aortic stenosis may influence the surgical approach and has been associated with higher perioperative morbidity (Citation6,Citation7). Thus it is of clinical importance to identify key covariates of ASH in patients with aortic stenosis to better understand this high-risk subpopulation. The present study adds to current knowledge on ASH in aortic stenosis by reporting prevalence and covariates of ASH and asymmetric LV hypertrophy in a large population of patients with asymptomatic aortic stenosis.
As demonstrated by our results, ASH is common among patients with asymptomatic aortic stenosis, being present in 22% of the study population. Few other studies have reported on the prevalence of ASH in aortic stenosis. Among them, Raj et al. reported a much lower prevalence of ASH, 2.5%, in among 200 patients with symptomatic aortic stenosis (Citation13). However, the present study population was older (45–85 vs 18–67 years) and the prevalence of hypertension higher, possibly explaining the different findings more than the difference in aortic stenosis severity between the studies (Citation13). Also among hypertensive study populations prevalence of ASH has differed from 4% to 30%, probably mostly reflecting differences in patient population characteristics (Citation2,Citation4,Citation13–15). Still, the majority have recognized ASH as an early and frequent structural adaptation in hypertension (Citation2–4) and some studies also found a preponderance of ASH in borderline hypertension (Citation3,Citation16).
Different mechanisms for development of ASH in hypertension have been described. In particular, because of its larger bending radius compared with the posterior wall, the septum may develop a greater tension during contraction, and therefore hypertrophy could appear earlier in the septum than in the posterior wall, suggesting that ASH represents earlier stages of hypertrophy in pressure overload conditions (Citation3). In our study population, no data on duration of hypertension was collected, but having ASH was associated with hypertension as well as higher total peripheral resistance and pulse pressure/stroke volume index independent of presence of concomitant LV hypertrophy and aortic stenosis severity, suggesting more systemic arterial dysfunction in patients with ASH.
In hypertensive populations, ASH has been accompanied by clinical characteristics potentially associated with increased cardiovascular risk (Citation2,Citation13,Citation17,Citation18). In our study, ASH was associated with higher LV mass, a well documented echocardiographic predictor of increased risk for cardiovascular events in hypertension (Citation12). LV mass has also been demonstrated to have a direct and continuous relation with cardiovascular morbid events in general population (Citation19) and LV hypertrophy to be an independent predictor for development of symptoms in patients with asymptomatic severe aortic stenosis (Citation20). In the SEAS study, we have previously demonstrated that having systemic hypertension is the main predictor for LV hypertrophy in asymptomatic aortic stenosis patients (Citation21), equal to the effect of 1 m/s higher peak transaortic jet velocity on LV mass in both genders (Citation22). Similar findings have been reported by Li et al. who found that the effect of mild-to-moderate aortic stenosis on LV hypertrophy was equivalent to that of mild-to-moderate hypertension (Citation23).
In the present study, patients with asymmetric LV hypertrophy were also characterized by a larger left atrial diameter. LA enlargement has previously been associated with higher risk of cardiovascular events in general population as well as in hypertensive patients with LV hypertrophy, in particular risk of atrial fibrillation and stroke (Citation17,Citation24). Furthermore, Dalsgaard et al. found that enlarged left atrial volume is common in asymptomatic aortic stenosis and associated with more severe valve disease independent of other covariates of left atrial size including hypertension, age, BMI, mitral regurgitation and LV hypertrophy (Citation18).
Finally, having asymmetric LV hypertrophy was also independently associated with lower LV ejection fraction in the present study population. Impaired LV systolic function has been identified as an independent preoperative risk factor for postoperative mortality after aortic valve replacement for aortic stenosis (Citation25) and represents a class I indication for valve replacement even in asymptomatic severe aortic stenosis (Citation26).
Conclusion
ASH is common in asymptomatic patients with aortic stenosis, present in 22% in the present study population. Hypertension was the main clinic covariate of ASH and associated with a clustering of other prognostically unfavorable factors, in particular arterial dysfunction and LV hypertrophy, and when asymmetric LV hypertrophy was present, also with LV systolic dysfunction and left atrial enlargement.
Declaration of interest: The SEAS Echocardiography Core Laboratory was supported by Merck/Schering-Plough Pharmaceuticals and Drs EG and KW have received honoraria from Merck/Schering-Plough Pharmaceuticals, for serving on the Scientific Steering Committee of the SEAS study 2002–8. The authors alone are responsible for the content and writing of the paper.
References
- Maron BJ, Edwards JE, Epstein SE. Disproportionate ventricular thickening in patients with systemic hypertension. Chest. 1978;73:466–470.
- Verdecchia P, Porcellati C, Zampi I, Schillaci G, Gatteschi C, Battistelli M, . Asymmetric left ventricular remodeling due to isolated septal thickening in patients with systemic hypertension and normal left ventricular masses. Am J Cardiol. 1994;73:247–252.
- Safar ME, Lehner JP, Vincent MI, Plainfosse MT, Simon AC. Echocardiographic dimensions in borderline and sustained hypertension. Am J Cardiol. 1979;44:930–935.
- Safar M, Benessiano JR, Hornysk AL. Asymmetric septal hypertrophy and borderline hypertension. Int J Cardiol. 1982; 2:103–108.
- Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, . Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol. 1997;29:630–634.
- Haering JM, Communale ME, Parker RA, Lowenstein E, Douglas PS. Krumholz HM, . Cardiac risk of noncardiac surgery in patients with assymetric septal hypertrophy. Anesthesiology. 1996;85:254–259.
- Mehta RH, Bruckman D, Dar S, Tsai T, Russman P, Karavite D, . Implications of increased left ventricular mass index on in-hospital outcomes in patients undergoing aortic valve surgery. J Thorac Cardiovasc Surg. 2001;122:919–928.
- Rossebø AB, Pedersen TR, Allen C, Boman K, Chambers J, Egstrup K, . Design and baseline characteristics of the simvastatin and ezetimibe in aortic stenosis (SEAS) study. Am J Cardiol. 2007;99:970–973.
- Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356.
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, . Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463.
- Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, . Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458.
- Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, . Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356.
- Raj MV, Srinivas V, Graham IM, Evans DW. Coexistence of asymmetric septal hypertrophy and aortic valve disease in adults. Thorax. 1979;34:91–95.
- Dunn FG, Chandraratna P, deCarvalho JG, Basta LL, Frohlich ED. Pathophysiologic assessment of hypertensive heart disease with echocardiography. Am J Cardiol. 1977; 39:789–795.
- Wicker P, Roudaut R, Haissaguere M, Villega-Arino P, Clementy J, Dallocchio M, Prevalence and significance of asymmetric septal hypertrophy in hypertension: An echocardiographic and clinical study. Eur Heart J. 1983;4 Suppl G:1–5
- Niederle P, Widimsky J, Jandova R, Ressl J, Grospic A. Echocardiographic assessment of the left ventricle in juvenile hypertension. Int J Cardiol. 1982;2:91–101.
- Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, . Left atrial size and risk of major cardiovascular events during antihypertensive treatment: Losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49:311–316.
- Dalsgaard M, Egstrup K, Wachtell K, Gerdts E, Cramariuc D, Kjaergaard J, Hassager C. Left atrial volume in patients with asymptomatic aortic valve stenosis (the Simvastatin and Ezetimibe in Aortic Stenosis study). Am J Cardiol. 2008;1;101: 1030–103425.
- Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–107.
- Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, . Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–3295.
- Rieck AE, Cramariuc D, Staal EM, Rossebø AB, Wachtell K, Gerdts E. J Hypertens. 2010;28:377–383.
- Cramariuc D, Rieck AE, Staal EM, Wachtell K, Eriksen E, Rossebø AB, . Factors influencing left ventricular structure and stress-corrected systolic function in men and women with asymptomatic aortic valve stenosis (a SEAS Substudy). Am J Cardiol. 2008;15;101:510–515.
- Li JK, Zhu JY, Nanna M. Computer modeling of the effects of aortic valve stenosis and arterial system afterload on left ventricular hypertrophy. Comput Biol Med.1997;27:477–485.
- Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, . Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: The Strong Heart Study (SHS). Am Heart J. 2006;151:412–418.
- Lund O, Flø C, Jensen FT, Emmertsen K, Nielsen TT, Rasmussen BS, . Left ventricular systolic and diastolic function in aortic stenosis. Prognostic value after valve replacement and underlying mechanisms. Eur Heart J. 1997;18:1977–198726.
- . 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic SurgeonsBonow RO, Carabello BA, Chatterjee K, de Leon AC Jr, Faxon DP, Freed MD, .; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. Circulation. 2008;118:523–661.