Abstract
Aim. The presence of peripheral arterial disease (PAD) in patients with other manifestations of cardiovascular disease identifies a population at increased risk of complications both during acute coronary events and on a long-term basis and possibly a population in whom secondary prevention of cardiovascular events should be addressed aggressively. The present study was aimed at providing a valid estimate on the prevalence of PAD in patients attending their general practitioner and having previously suffered a cardio- or cerebrovascular event. Patients and methods. 1000 patients with a previous cardiovascular event were screened and PAD was considered present when the ankle–brachial index (ABI) of systolic blood pressure was less than 0.90 using the current recommended technique. Results. 965 (659 men) patients met the inclusion criteria and had detectable systolic blood pressures on the arms and ankles. Mean age was 70±8 years, 77% were current or previous smokers, and 188 patients were diabetics. The medical history included stroke in 392, transitory ischemic attacks in 77, acute coronary syndrome in 298, and ST-elevation myocardial infarction in 253. Brachial systolic and diastolic blood pressures were 139±18 mmHg and 79±12 mmHg, respectively. Total and LDL-cholesterols were 4.5±1.0 mmol/l and 2.4±0.8 mmol/l, respectively. 625 patients were without PAD, 322 had mild to moderate PAD and 18 had severe PAD. The overall prevalence of PAD was 35.3%. Conclusion. In patients with previous coronary or cerebrovascular events, PAD occurs with a much higher prevalence than previously estimated. It is suggested that screening for PAD is justified and that it should be carried out in these patients in order to regulate the possible lifestyle and medical intervention.
Introduction
Peripheral arterial disease (PAD) refers to flow disturbances in all arteries other than the coronary and intracranial vessels but is commonly used synonymously with atherosclerotic obstruction in the circulation of the lower limbs. PAD is associated with a significant increase in cardiovascular risk as well as in the prevalence of myocardial infarction, stroke and congestive heart failure (Citation1,Citation2). Patients with PAD, even in the absence of a history of myocardial infarction or ischemic stroke, have approximately the same relative risk of death from cardiovascular causes as do patients with a history of coronary or cerebrovascular disease (Citation3). PAD is usually diagnosed on the basis of history and non-invasive comparison of blood pressures at the ankle and brachial level. The prevalence of symptomatic PAD with manifestations such as intermittent claudication has been estimated to range between 2% and 7% (Citation4). The ratio between asymptomatic and symptomatic intermittent claudication is found to be between 1:1 and 6:1, and it is estimated that between 25% and 30% of patients with a coronary or significant cerebrovascular disease also have PAD (Citation5). The presence of PAD – be it symptomatic or asymptomatic – identifies a population at increased risk of complications both during acute coronary events (Citation6) and on a long-term basis (Citation7).
The purpose of the present study was to derive a more precise estimate of the prevalence of PAD in patients with previous coronary or cerebrovascular events attending their general practitioner.
Patients and methods
Study population
A computer program performed a random selection of sites based on a list of all general practitioners (GPs) in Denmark to ensure that centers were geographically and demographically representative. A total of 3500 GPs are distributed over 16 municipalities. The 16 municipalities were divided into two groups (A and B) according to number of inhabitants. Group A consisted of the five biggest municipalities in Denmark with ≥400,000 inhabitants and group B consisted of the remaining counties/municipalities with <400,000 inhabitants. Thirty-one GPs were randomly selected between the GPs in group A and each of these sites should enroll 25 patients. Seventy-eight GPs were randomly selected the GP's in group B and each of these sites should enroll 10 patients. Accordingly, 775 patients were to be enrolled from each group. This sample size of 1550 was based on an estimate of a true prevalence of 20%. However, during the course of the study, an updated estimate of about 36–40% prevalence, suggested a sample size of a 1000 subjects would yield a power of 83%. Thus, it was decided to stop enrolment after 6 months when 1000 subjects had been included. Because of slow enrollment, it was decided to include additional sites leading to a total of 34 and 82 in groups A and B, respectively. The study was monitored by an external clinical research organization. All 116 sites were visited once and 25 (21.6%) were randomly chosen for full source verification in a total of 269 patients (26.9%). Patients were enrolled on a consecutive basis according to the following inclusion criteria: documented coronary or cerebrovascular atherothrombotic event, and age 55 years or above. The only exclusion criteria were immeasurable ankle pressure and any circumstances preventing the subjects from signing the informed consent. The study was approved by the local ethics committee and the patients gave written and orally informed consent to participate.
Blood pressure measurements
Systolic blood pressure at the ankle level was estimated by standard Doppler technique after 10 min of rest in the supine position (Citation5). Pressure readings were obtained from both the anterior tibial/dorsalis pedis artery and the posterior tibial artery. All investigators were offered training in the blood pressure measurements. The brachial pressure was measured by standard, semiautomatic oscillometric equipment in both arms. ABI was calculated by using the highest pressure reading from the two measuring sites in the ankle with the lowest mean value of systolic blood pressures divided by the highest brachial systolic blood pressure. An ABI <0.9 was considered diagnostic of PAD.
Clinical data
The following data were obtained in all patients: date of birth, gender, general medical history, medical history regarding previous atherothrombotic event, and present treatment. The following data were obtained if available and not older than 6 months: blood lipid levels, smoking history, height and weight. If used at time of entry, the following medical treatment was recorded: beta-blockers, diuretics, calcium-channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II antagonists (AIIA), dipyridamol, statins, acetylsalicylic acid, clopidogrel and glucose-lowering treatment.
Statistical analysis
All subjects who had a measurable ABI were included in the evaluation and all data in this report were evaluated by descriptive statistics only. No adjustments for covariates were made. All subjects with observed values were included in the tabulations. Subjects with missing data were not included in the tabulation concerned. Comparisons of dichotomous data were done by the chi-square test with a two-sided significance level of 5% unless otherwise indicated. No interim analysis was performed in this study. In total, 116 centers (GP clinics) were involved in this study. All participating centers are listed on last page.
Results
A total of 1000 patients met the inclusion criteria but 35 had to be excluded from the analysis, as not all protocol-related information was provided by the investigators and of these, 11 patients had withdrawn from the study for various reasons. The per-protocol population therefore consisted of 965 patients – 306 women (31.7%) and 659 men (68.3%). The mean (±SD) age was 70.0± 7.9 years. The mean height was 171±8.3 cm, mean weight was 79.5± 14.5 kg resulting in a mean body mass index (BMI) of 27.1±4.1 kg/m2. The previous cerebrovascular or coronary events leading to inclusion were stroke in 392, acute coronary syndrome in 298, ST-elevation myocardial infarction in 253, transitory ischemic attack in 77 and 10 events of unknown origin.
Diabetes was present in 188 patients, primarily type-II (88%). A total of 918 patients responded to the question regarding smoking habits, and of these 77% were current or previous smokers whereas 211 patients had never smoked. The mean systolic and diastolic blood pressures were 139±18.3 and 79± 11.7 mmHg, respectively, at a mean heart rate of 70± 10.5 beats/min. The mean ankle–brachial index (ABI) was 0.93 ranging from 0.22 to 1.61. Recent values for lipids could be obtained in 632 patients and gave the following results: total cholesterol 4.52 ±0.99 mmol/l, high-density lipoprotein (HDL)-cholesterol 1.48±0.44 mmol/l), low-density lipoprotein (LDL)-cholesterol 2.40±0.82 mmol/l and triglycerides 1.46±0.86 mmol/l.
Demographics, blood pressure and cholesterol values according to the ABI classes are given in . In 625 patients (64.8%), the ABI score was ≥0.90, 322 (33.4%) had a score <0.90 but ≥0.40, while 18 patients had a score of <0.40 (1.9%). The overall prevalence of an abnormal ABI score was 35.3%. The prevalence of PAD was 37.3% in women and 34.3% in men but this difference was not significant.
Table I. Demographics of patients according to level of ankle–brachial index (ABI).
The 625 patients in the normal group had a mean ABI score of 1.05 (95% confidence interval: 1.04–1.05). The 322 patients with moderately reduced ankle pressure had a mean ABI score of 0.73 (0.72–0.74), while the 18 patients with severely reduced ankle pressure had a mean score of 0.34 (0.32–0.36).
Of those with an ABI less than 0.40, 89% were smokers, whereas 82% were present or previous smokers in the group with ABI between 0.40 and 0.90, and 74% in the group with ABI values above 0.90. The frequency of current or previous smokers was significantly higher in those with PAD than in those with a normal ABI. Of the 182 diabetic patients included, 57% had a normal ABI, 39% had a moderately reduced ABI and 4% had a severely reduced ABI. The frequency of PAD was significantly higher in patients with diabetes.
Stroke was the inclusion criterion for 37%, 39% and 38% among the patients classified as having severe PAD, moderate PAD and normal ABI, respectively. Transitory ischemic attack was the inclusion criterion for 11%, 6% and 8% among the patients classified as having severe PAD, moderate PAD and normal ABI, respectively. Acute coronary syndrome was the inclusion criterion for 32%, 30% and 29% among the patients classified as having severe PAD, moderate PAD and normal ABI, respectively. Myocardial infarction with ST-elevation (STEMI) was the inclusion criterion for 21%, 25% and 25% among the patients classified as having severe PAD, moderate PAD and normal ABI respectively. We could not demonstrate any significant differences in the frequencies of the various inclusion criteria between those with PAD and those with a normal ABI.
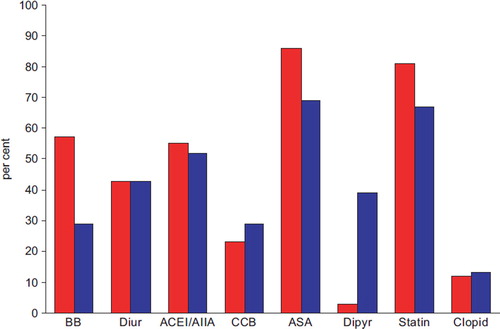
The distribution of patient medication is given in . Overall, 47% of the included patients received a beta-blocker, 45% were on diuretics, 57% received an ACEI or an AIIA, 27% were on CCBs, 21% received dipyridamol, 83% were treated with low-dose acetylsalicylic acid, 13.2% were on clopidogrel and 79% received a statin. Patients with cerebrovascular end-points as inclusion criteria received significantly less beta-blockers (p<0.001), statins (p<0.001), aspirin (p<0.001) and more dipyridamol than those with coronary end-points. The use of diuretics ACE/AIIA, CCBs and clopidogrel did not differ between groups. We could not demonstrate any significant differences in medication between those with PAD and those with a normal ABI.
Figure 1. Distribution of medication in patients with previous coronary or cerebrovascular events. The bars show the percentage of patients with either coronary (red, n=516) or cerebrovascular (blue, n=439) inclusion events receiving beta-blockers (BB), diuretics (Diur), angiotensin converting enzyme inhibitors/angiotensin II antagonists (ACEI/AIIA), calcium-channel blockers (CCB), acetylsalicylic acid (ASA), dipyridamol (Dipyr), statin, or clopidogrel (Clopid).
Discussion
Measurement of brachial and ankle blood pressure with subsequent derivation of the ABI is a highly sensitive and specific method for the detection and quantification of PAD. The coexistence of coronary or cerebrovascular disease and PAD has been demonstrated in several studies and PAD has been shown to carry a poor prognosis in itself. In the present study, we have shown that the prevalence of PAD is very high in outpatients who have already suffered a coronary or a cerebrovascular event and we suggest that a routine determination of ABI could have important therapeutic implications. Our data also point to a possible therapeutic gap particularly in patients with cerebrovascular disease and in those with widespread atherosclerosis.
Epidemiological studies in subjects at or over 60 years of age have shown that 2% (Citation4) to 6% (Citation8) of the population have intermittent claudication, but it has also been suggested that the prevalence is three- to fourfold higher, when tests such as ankle pressure measurements are applied (Citation4). Indeed, in a recent study, we found the prevalence of PAD to be approximately 12% in patients aged 55 years or more attending their general practitioner for any cause when the ABI was used for the diagnosis (Citation9). Similarly, a large German study found a prevalence of PAD of 20% in men and 17% in women over the age 65 years attending their general practitioner (Citation10). Estimates on the prevalence of PAD are strongly influenced by the methods applied with the use of questionnaires based on symptoms having the lowest sensitivity and the ABI determinations as recommended by the American Heart Association (Citation5) having the best compromise between sensitivity and specificity (Citation11). The estimated prevalence of PAD is also dependent upon the age of the cohort studied, the underlying atherosclerosis risk factor profile of the cohort, and the presence of other concomitant manifestations of atherosclerosis. The coexistence of coronary artery disease (CAD), cerebrovascular disease (CVD) and PAD is illustrated by the study of Ness & Aronow (Citation12) in geriatric patients, where a prior stroke was associated with a prevalence of CAD and PAD of 56% and 28%, respectively, and a diagnosis of PAD was coupled to a prevalence of CAD and CVD of 68% and 42%, respectively. A recent study comparing the results of coronary angiography to ABI-levels it was shown that younger, non-obese patients with reduced ABI had a 52% probability of having coronary lesions compared 1% in those with normal ABI (Citation13). In the A Global Atherosclerosis Assessment (AGATHA) study including almost 9000 diseased or at-risk patients, PAD was found to be present in 20% of patients with CAD and in 26% of patients with CVD (Citation14). Based on previous studies, and ours, there seems little doubt that affection of one vascular bed is strongly associated with concomitant disease in other vascular beds. It also seems clear that the presence of PAD is more predictive of CAD and CVD than vice versa, probably related to the higher transmural pressure in the vasculature of the lower extremities increasing the possibility of more pronounced atherosclerosis. Our study has shown that in order to identify one patient with PAD among patients with a previous coronary or cerebrovascular event, it would only be necessary to screen three patients. Based on the study by Doubeni et al. (Citation15), identifying one patient with PAD in this context would only require 45 min of ABI-testing.
A recent study in patients with both PAD and CAD has shown that the inflammatory response elicited from the lower limbs during exercise may deteriorate coronary endothelial function and has pointed to the possibility that PAD, besides being a risk marker, could promote the progression of coronary artery disease. If this negative synergy could be further substantiated, it would mean that such patients should be the target of more aggressive antiatherosclerotic therapy.
According to present guidelines (Citation5), antiplatelet and statin treatments are indicated for all patients with PAD, as are blood pressure lowering drugs, should hypertension be present. The medical records from our patients have shown a reasonable high usage of statins and low-dose acetylsalicylic acid but have also pointed to a possible care gap in the patients with CVD and to the fact that the intensity of medical treatment was not increased in patients with coexisting PAD. Both in the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) and the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial, patients with documented prior MI, ischemic stroke, or symptomatic PAD appeared to derive significant benefit from dual antiplatelet therapy with clopidogrel plus low-dose acetylsalicylic acid (Citation16,Citation17). The relative low use of clopidogrel in our patients probably reflects that the study was undertaken before the results of the mentioned studies were available.
Conclusions
It is concluded that PAD frequently coexist with coronary and cerebrovascular disease to such a degree that it warrants screening. It is concluded that with the given prevalence the identification of PAD requires only a limited amount of time and could be performed in general practice. It is also concluded that a therapeutic gap may exist in our current practice as the presence of PAD does not seem to have an impact on medical treatment even though this condition may indicate of a worse prognosis and hence, a demand for more aggressive therapy.
Acknowledgements
The study was funded by Bristol-Myers Squibb and Sanofi-Aventis.
Disclosures: All authors received economic compensation from Bristol-Myers Squibb and Sanofi-Aventis for their work associated with the study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Zheng ZJ, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, . Associations of ankle–brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125.
- Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: The Framingham Study. Arch Intern Med. 2003;163:1939–1942.
- Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, . Ankle–arm index as a predictor of cardiovascular disease and mortality in the Cardiovascular Health Study. The Cardiovascular Health Study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–545.
- Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71: 510–515.
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, . ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report. Circulation. 2006;113:e463–e654.
- Bertomeu V, Morillas P, Gonzalez-Juanatey JR, Quiles J, Guindo J, Soria F, . Prevalence and prognostic influence of peripheral arterial disease in patients >or=40 years old admitted into hospital following an acute coronary event. Eur J Vasc Endovasc Surg. 2008;36:189–196.
- Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, . Long-term prognosis of patients with peripheral arterial disease: A comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51: 1588–1596.
- Schroll M, Munck O. Estimation of peripheral arteriosclerotic disease by ankle blood pressure measurements in a population study of 60-year-old men and women. J Chronic Dis. 1981;34:261–269.
- Mehlsen J, Wiinberg N, Bruce C. Oscillometric blood pressure measurement: A simple method in screening for peripheral arterial disease. Clin Physiol Funct Imaging. 2008;28:426–429.
- Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange SF, . High prevalence of peripheral arterial disease and co-morbidity in 6880 primary care patients: Cross-sectional study. Atherosclerosis. 2004;172:95–105.
- Lange SF, Trampisch HJ, Pittrow D, Darius H, Mahn M, Allenberg JR, . Profound influence of different methods for determination of the ankle brachial index on the prevalence estimate of peripheral arterial disease. BMC Public Health. 2007;7:147.
- Ness J, Aronow WS. Prevalence of coexistence of coronary artery disease, ischemic stroke, and peripheral arterial disease in older persons, mean age 80 years, in an academic hospital-based geriatrics practice. J Am Geriatr Soc. 1999;47: 1255–1256.
- Gabriel SA, Serafim PH, Freitas CE, Tristao CK, Taniguchi RS, Beteli CB, . Peripheral arterial occlusive disease and ankle–brachial index in patients who had coronary angiography. Rev Bras Cir Cardiovasc. 2007;22:49–59.
- Fowkes FG, Low LP, Tuta S, Kozak J. Ankle–brachial index and extent of atherothrombosis in 8891 patients with or at risk of vascular disease: Results of the international AGATHA study. Eur Heart J. 2006;27:1861–1867.
- Doubeni CA, Yood RA, Emani S, Gurwitz JH. Identifying unrecognized peripheral arterial disease among asymptomatic patients in the primary care setting. Angiology. 2006;57: 171–180.
- Ringleb PA, Bhatt DL, Hirsch AT, Topol EJ, Hacke W. Benefit of clopidogrel over aspirin is amplified in patients with a history of ischemic events. Stroke. 2004;35:528–532.
- Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, . Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–1988.