Abstract
Aims. The aim of the present study was to evaluate the influence of gestational hypertension on hemodynamics and cardiovascular autonomic regulation at rest and their responses to head-up tilt (HUT). We prospectively studied 56 pregnant women (28 with gestational hypertension and 28 healthy pregnant women) during the third trimester of pregnancy and 3 months after pregnancy. Major findings. In women with pregnancy-induced hypertension, compared with control women, there were significant differences in hemodynamics and in markers of cardiovascular regulation (p < 0.05 to p < 0.001). Postural change from the supine to the upright position was associated with significant changes in hemodynamic responses in both groups during pregnancy (from p < 0.05 to p < 0.001). Regulatory response to HUT in both groups was characterized with a decrease in HF power and increase in LF/HF ratio (from p < 0.01 to p < 0.001). Responses to HUT in total power and VLF power were attenuated in hypertensive pregnancies (p < 0.001 to p < 0.01, respectively, vs control group). Conclusions. Our results suggest that autonomic cardiovascular regulation may not play a major role in women with gestational hypertension . The lack of irreversible changes in autonomic nervous function in hypertensive women appeared to be a feature of gestational-induced hypertension.
Introduction
Normal pregnancy requires profound maternal hemodynamic changes and extracellular volume expansion to create adequate uteroplacental circulation for the growing fetus (Citation1). Hemodynamic changes in normal pregnancy include a decrease in mean arterial pressure and systemic vascular resistance, and an increase in circulating volume, heart rate and cardiac output (Citation2). During puerperium, hemodynamic changes have been found to be relatively stable 12 weeks after the delivery although heart rate, cardiac index and blood pressure fall below the third trimester level. Total peripheral resistance increases by the 6th week after the delivery and remains at that level up to 12 weeks postpartum (Citation3,Citation4). Overall, these findings suggest relatively rapid recovery from the state of the increased circulating blood volume and preload peculiar to pregnancy. Autonomic nervous system plays a central role in the adaptation of the cardiovascular system to various hemodynamic needs and is the principal system involved in short-term cardiovascular control (Citation5). Normal pregnancy is known to be associated with parallel decrease of parasympathetic and sympathetic activity at rest. Presumably, blunted sympathovagal activation and hemodynamic stability contribute to the maintenance of optimal uteroplacental blood flow and the well-being of the fetus (Citation6).
The most common definitions concerning hypertension in pregnancy are gestational hypertension, pre-eclampsia, chronic hypertension and superimposed pre-eclampsia (Citation7). In hypertensive pregnancies, women have been found to have significantly lower blood and plasma volume than women with uncomplicated pregnancies. The difference in plasma volume is thought to be related to endothelial dysfunction (Citation8). Discrepancies between longitudinal and cross-sectional studies regarding cardiac output and peripheral vascular resistance in hypertensive pregnancies compared with normotensive pregnancies exist. Some investigators report significantly higher cardiac outputs and heart rates in hypertensive pregnancies, but no differences in total peripheral vascular resistance and stroke volumes (Citation9,Citation10). In some cross-sectional studies, cardiac output has been found to be significantly lower and systemic vascular resistance higher than in normotensive pregnancies (Citation11,Citation12).
Autonomic dysregulation appears to be closely involved in cardiovascular complications associated with gestational hypertension. The association between alterations in autonomic cardiovascular function and the development of hypertension in pregnancy has been investigated for some time. There are few studies that have used spectral analysis of blood pressure and heart rate variability (HRV) to evaluate the function of autonomic nervous system in pre-eclampsia and gestational hypertension, showing conflicting results ranging from decreased vagal control of the heart rate to increased sympathetic and parasympathetic control of heart rate and blood pressure (Citation13,Citation14). The orthostatic test has been used as a simple, reliable and reproducible as well as non-invasive method for studying the autonomic nervous control of circulation. Furthermore, assessment of cardiovascular variability and baroreflex sensitivity (BRS) in association with postural changes has been found to be useful in evaluation of dynamic regulatory capacity and characterization of adaptive strategies in maintaining blood pressure homeostasis (Citation15).
We hypothesized that gestational hypertension may be associated with early autonomic dysfunction and impaired cardiovascular autonomic regulation. We therefore evaluated changes in central hemodynamics and cardiovascular variability in response to orthostatic challenge in gestational hypertension in comparison with healthy pregnant women.
Materials and methods
Subjects
We prospectively studied 56 pregnant women (28 with pregnancy-induced hypertension or pre-eclampsia and 28 healthy pregnant women) during third trimester of pregnancy and 3 months after pregnancy. The patients were recruited from the Kuopio University Hospital maternity clinic, where they were visited for gestational hypertension or pre-eclampsia. The control subjects were outpatients with gestational age similar to those of the patients with gestational hypertension. Gestational hypertension was defined as a systolic blood pressure level ≥140 mmHg or a diastolic blood pressure level ≥90 mmHg after 20 weeks of pregnancy in women with previously normal blood pressure and with or without proteinuria. Classification based on three separate measurements. Control subjects remained normotensive throughout their pregnancies. Pregnant women with superimposed pre-eclampsia were excluded from study (for example women with renal disease or essential hypertension). Measurements for the study performed in the morning (from 08.00 to 11.30 h). The maternal electrocardiogram (Rigel Multicare 302, Morden, Surrey, UK) and arterial blood pressure (Finapres digital plethysmograph, Ohmeda Inc., Englewood, CO, USA) were measured non-invasively beat to beat throughout the study protocol during and after pregnancy. The study was approved by the Ethical Committee of the Kuopio University Hospital, and all participants gave informed consent.
Assessment of hemodynamics and cardiovascular autonomic regulation
Recordings. The maternal electrocardiogram (Rigel Multicare 302) and arterial blood pressure (Finapres digital plethysmograph) were measured non-invasively beat to beat throughout the study protocol (as described earlier; 6). Continuous blood pressure recording was performed from the middle finger of the right hand. During the recordings, subjects’ right hand was immobilized in a mitella adjusting finger cuff to the level of fourth intercostal space, which corresponds the level of the right atrium. All recordings and data analyses were performed with an IBM PC-compatible microcomputer with WINACQ/WINCPRS software (Absolute Aliens Oy, Turku, Finland).
Protocol. At first, 10-min recordings were performed at rest in the supine (left-lateral) position, while subjects were asked to breathe according to a metronome at 0.2-Hz frequency with their normal tidal volume to enable assessment of baseline hemodynamics and autonomic regulation. Then the protocol continued with the head-up tilt (HUT) test without controlling breathing frequency to avoid occurrence of cardioneurogenic reflex syncopal reactions in the upright position.
Hemodynamic response related to change of position from supine to upright position is a standardized, physiological and clinically relevant stimulus that challenges the cardiovascular regulation. By the performance of HUT, it is possible to assess the dynamic capacity of the regulatory systems (Citation16). In the HUT test, ECG and blood pressure signals were recorded for a period of 10 min in the supine position (left-lateral position) on the tilt table. Thereafter, the tilt table (Läätek Ltd, Kaarina, Finland) was inclined within 5 s to 70° upright position for another 10-min recording, and then returned back to the horizontal position.
Assessment of hemodynamics. Stroke volume was assessed from the non-invasive blood pressure signal by using the arterial pulse contour method, which is modified from the model flow method (Citation16). Cardiac output was calculated from the equation: cardiac output = (heart rate) × (stroke volume). Peripheral resistance was calculated as: total peripheral resistance = (mean arterial pressure)/(cardiac output). Mean heart rate, systolic, diastolic and mean arterial blood pressure, stroke volume, cardiac output and total peripheral resistance were assessed during stationary 5-min periods in the supine and upright position free from ectopic beats and artifacts.
Assessment of HRV. Spectral estimates of R–R interval and systolic blood pressure were obtained from the same regions. Fast Fourier Transform was used to obtain power spectral estimates of HRV and systolic blood pressure variability (SBPV). Total power in the frequency range 0–0.40 Hz was divided into very low frequency (VLF; 0.0–0.04 Hz), low frequency (LF; 0.04–0.15 Hz) and high frequency (HF; 0.15–0.40 Hz) bands. Signal powers of each band were calculated as integrals under the respective power spectral density functions, and expressed in absolute units (ms2 or mmHg2). Cardiovascular variability was analyzed following the recommendations of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Citation17).
Statistical analysis
Because of the skewed distribution, HRV and BRS were analyzed after logarithmic transformation and their changes during HUT after rank-transformation. Statistical significance of difference between gestational hypertension and control group was analyzed using the t-test or analysis of covariance (ANCOVA). Time-related (supine vs upright; pregnancy vs 3 months after parturition) changes were analyzed using multivariate analysis of variance (MANOVA) for repeated measures. Calculations were performed using SPSS for Windows software (SPSS Inc., Chicago, IL, USA). Data are shown as means ± SEM (standard error mean). p < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study subjects are summarized in . Women of both groups were of a similar age and gestational age at the time of enrollment. Women with gestational hypertension had a higher body mass index (BMI) during pregnancy compared with the reference group (p < 0.001). Overall, women with gestational hypertension gained more weight than other groups during pregnancy (15.3 kg) and they were also unable to regain their pre-pregnancy weight after delivery. In hypertensive women the mean gestational age at delivery and children's birth weight were significant lower from those of the control group (p < 0.001).
Table I. Clinical characteristics in patients with gestational hypertension and healthy subjects.
Hemodynamic and cardiovascular changes during pregnancy and puerperium
There were marked differences in parameters reflecting baseline hemodynamics and autonomic regulation between the women during pregnancy compared with the same women 3 months after pregnancy at rest (controlled breathing at 0.2 Hz frequency) (). Systolic blood pressure (p < 0.001), diastolic blood pressure (p < 0.001) and peripheral resistance (p < 0.001) were higher, while stroke volume (p < 0.001) were lower during pregnancy than 3 months after delivery. There were no pregnancy-related effects seen in cardiac output. Adjustment for BMI did not change the statistical significance of the hemodynamic results with the exception of peripheral resistance during pregnancy, which came statistically significant between cases and controls after adjustment (data not shown). Correspondingly, pregnancy was found to modulate HRV and baroreflex function assessed at rest during fixed paced breathing (). Total power and its spectral components were all statistically significantly decreased during pregnancy. Furthermore, BRS was found to become slightly decreased during pregnancy according to αLF values (p < 0.01).
Table II. Hemodynamics and cardiovascular autonomic regulation at rest (controlled breathing at 0.2-Hz frequency) during pregnancy and 3 months after delivery in patients with pregnancy induced hypertension and healthy subjects.
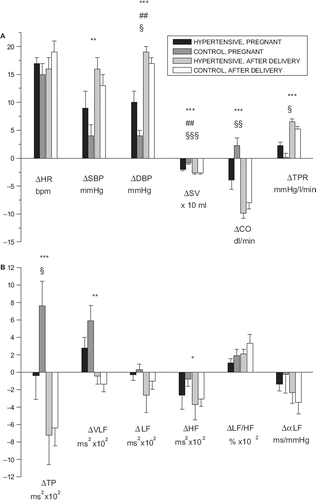
Circulatory responses to HUT are shown in . Pregnancy-related effects were seen in HUT responses of systolic blood pressure (p < 0.01) and diastolic blood pressure, stroke volume, cardiac output and total peripheral resistance (p < 0.001 for all). Cardiovascular autonomic responses to HUT are shown in . Pregnancy-related effects were seen in HUT responses of total power (p < 0.001), in VLF component (p < 0.01) and slightly in HF component (p < 0.05).
Figure 1. (A) Circulatory and cardiovascular responses to head-up tilt (HUT) in gestational hypertensive and control women in third trimester during pregnancy and postpartum. Values are means±SE. Significances: effect of pregnancy * p < 0.05, ** p < 0.01 and *** p < 0.001; group interaction ##p < 0.01; difference between hypertensive and control groups during pregnancy §p < 0.05, §§p < 0.01 and §§§p < 0.001. Abbreviations: ΔHR, response in heart rate; ΔSBP, response in systolic blood pressure; ΔDBP, response in diastolic blood pressure; ΔSV, response in stroke volume; ΔCO, response in cardiac output; ΔTPR, response in total peripheral resistance. (B) Regulatory responses to HUT in gestational hypertensive and control women in third trimester during pregnancy and postpartum. Values are means ± SE. Significances: effect of pregnancy * p < 0.05, **p < 0.01 and *** p < 0.001; difference between hypertensive and control groups during pregnancy §p < 0.05. Abbreviations: ΔTP, response in total spectral power; ΔVLF, response in very low-frequency spectral component; ΔLF, response in low-frequency spectral component; ΔHF, response in high-frequency spectral component; ΔLF/HF, response in LF/HF ratio; ΔαLF, response in cross-spectral baroreflex sensitivity index αLF.
Hemodynamic and cardiovascular changes in normal and hypertensive pregnancies
There were marked differences in parameters reflecting baseline hemodynamics between the women with gestational hypertension and control pregnant women at rest (controlled breathing at 0.2 Hz frequency) (). Systolic blood pressure, diastolic blood pressure, stroke volume and cardiac output were higher (p< 0.001 for all), whereas heart rate (p < 0.05) was lower during pregnancy in hypertensive pregnancies. Three months after delivery, women with gestational hypertension still had higher systolic blood pressure (p < 0.05) and diastolic blood pressure (p < 0.01) compared with control women. Pregnancy was not found to modulate HRV and baroreflex function assessed at rest during fixed paced breathing (). Only HF component was slightly higher in hypertensive pregnant women compared with normotensive pregnant women (p <0.05). There were no statistical differences in total power, VLF component, LF component or BRS between groups during pregnancy or 3 months after delivery.
Circulatory responses to HUT are shown in . During pregnancy, statistically significant differences between the women with gestational hypertension and the control women were seen in HUT responses of diastolic blood pressure, total peripheral resistance (p < 0.05), cardiac output (p < 0.01) and stroke volume (p < 0.001). Change in position was associated with a greater increase in blood pressure and peripheral resistance in hypertensive than in normal pregnancies. Furthermore, decreases of stroke volume and cardiac output in response to HUT were seen in hypertensive women, whereas in normal pregnancies, a slight decrease in stroke volume in response to HUT was not accompanied by a decrease in cardiac output. Cardiovascular autonomic responses to HUT are shown in . The effect of belonging to the study or control group was seen in HUT responses of total power (p < 0.05) but not in other regulatory parameters during or after pregnancy.
Discussion
In the present study, the specific role of gestational hypertension in autonomic regulation was studied. Gestational hypertension, representing a pre-cardiovascular condition, is of particular interest, as it allows us to evaluate whether early autonomic dysfunction is present in such women. The main finding of the present study was that pregnant women respond to autonomic nervous system control differently from non-pregnant women and both pregnancy and gestational hypertension changed hemodynamic functions remarkably. Women with gestational hypertension showed no signs of significant autonomic dysregulation during or after pregnancy. In fact, HF power was higher during pregnancy in women with pregnancy-induced hypertension suggesting well-preserved vagal modulation of the heart rate and attenuated parasympathetic deactivation in pregnancy. We conclude that it is unlikely that autonomic cardiovascular regulation plays a major role in the development of gestational hypertension.
At rest, in the present study, gestational hypertension appeared to be associated with a significant increase of blood pressure, stroke volume and cardiac output. However, in women with pregnancy-induced hypertension, the heart rate was lower during pregnancy than in control pregnant women. If blood pressure is high enough, baroreflex starts to decrease the heart rate to maintain vascular homeostasis. In our study, there were no significant differences in peripheral resistance between the study and control groups. There is a clear disagreement in the literature regarding how pregnancy-induced hypertension affects hemodynamics. One longitudinal study reported that pre-eclamptic parturients had significantly higher cardiac outputs, but no differences in total peripheral vascular resistance compared with normotensive pregnant women before clinical diagnosis of pre-eclampsia (Citation9). Our results are in line with these findings but they are inconsistent with some other studies, which have found a higher heart rate and total peripheral vascular resistance (Citation8) and lower cardiac output in hypertensive pregnant women compared with normotensive control women (Citation10,Citation11). Maybe the higher cardiac output tries to keep placental blood flow high enough to secure fetal nutrition and growth. Three months after delivery, blood pressure was still found to be high in women with gestational hypertension compared with control women.
In the literature, a hyperdynamic model of hemodynamics in pre-eclampsia has also been proposed, implying that pre-eclamptic women who have low cardiac output all have high vascular resistance, but pre-eclamptic women with high cardiac output have normal to low systemic vascular resistance (Citation22). The present results are in line with hyperdynamic hemodynamics showing high cardiac output and normal systemic vascular resistance. One limitation in our study is that stroke volume was assessed using the arterial pulse contour method based on solid physical principles and less solid physiological models, which involve substantial computations. However, it has been shown that the pulse contour method suffices in tracking the changes in stroke volume and cardiac output, but for absolute values it is not reliable (Citation23,Citation24).
At rest, normal human pregnancy appeared to be associated with a decrease of total power, as well as all of its components VLF, LF and HF powers in frequency domain analysis. Vagal activity is the major contributor of the absolute levels of HF, LF and total power, whereas normalized LF and LF/HF ratio represent sympathetic activity. VLFs are related to various regulatory mechanisms, such as chemoreception and temperature regulation (Citation25–27). Collectively, the present frequency domain analysis suggested that normal human third trimester pregnancy was characterized rather by parasympathetic deactivation than by increased sympathetic activity under an unstimulated condition. Furthermore, the present study shows that this change is similar in pregnancies affected by gestational hypertension and in unaffected pregnancies.
At rest, in our study women with pregnancy-induced hypertension had an increase in HF power compared with control pregnant women suggesting parasympathetic activation. There were no significant changes in other components of total power between the study and control groups. Studies concerning autonomic nervous function in pre-eclampsia and gestational hypertension have reported inconclusive results. A study that used spectral analysis showed a significant reduction of high frequency of inter-beat interval variability, indicating that pre-eclampsia was associated with decreased vagal control of the heart (Citation24), whereas Ekholm et al. (Citation27) have shown increased heart rate and blood pressure variability in women with pre-eclampsia, suggesting an increase in both sympathetic and parasympathetic components. The present results concerning HF component are in line with the latter study and the discordant results could be related both to the different conditions of breathing (spontaneous or controlled breathing) and to the autoregressive method chosen for the spectral analysis (Citation28).
In both groups, HUT induced changes in hemodynamics, which were characterized by an increase in blood pressure, total peripheral vascular resistance and a decrease in cardiac output and stroke volume. Heart rate remained virtually unchanged during pregnancy and after pregnancy in the study and control groups. The high cardiac output of pregnancy was maintained in the standing posture, because of the optimized interplay between the responses to the postural challenge of the increased preload and the reduced afterload (Citation29). As a response to HUT in normal pregnant women, total power and VLF power increased, while its LF component remained virtually unchanged and HF component decreased during pregnancy. In women with pregnancy-induced hypertension, total power was decreased during pregnancy compared with control pregnant women. After pregnancy, the total power decreased together with its LF, HF and VLF components. However, LF/HF ratio increased in both groups and time points, suggesting a shift towards parasympathetic predominance as a response to HUT.
Altogether the response to HUT was comparable in gestational hypertension (only TP was lower in hypertensive women during pregnancy) and normal pregnancies. After HUT, blood pressure variability showed no differences between the women with pregnancy-induced hypertension and control women. Molin et al. (Citation28) have reported opposite results concerning regulatory responses to HUT, showing that pre-eclamptic women had a reduction in the LF and an increase in the HF components of the HRV and the LF/HF ratio was also reduced compared with non-pregnant women. Interestingly, studies looking at plasma norepinephrine concentrations in response to tilting have yielded similar results, with pregnant women having reduced sympathoadrenal responses to orthostatic stress (Citation26,Citation30). It seems that during normal pregnancy after HUT, there is less need to use the parasympathetic deactivation to maintain stable hemodynamics. The increased intravascular plasma volume during pregnancy probably explains it (Citation5).
The subjects in our study served as their own non-pregnant controls. This kind of a longitudinal study gave, however, a more reliable understanding of the changes in circulatory control during pregnancy than cross-sectional studies. The discrepancies between studies in hemodynamics and autonomic vascular regulation during and after pregnancy can partly be explained by the different methods used. The posture of a subject when hemodynamic parameters are measured could also explain disagreement between studies.
The autonomic nervous system appears to play an important role in hemodynamic functions during pregnancy, as evidenced by reduced circulatory and sympathoadrenal responses to orthostatic stress. The current study shows that the physiological decrease in HRV during HUT is absent in patients with gestational hypertension. The maladaptation of the cardiovascular control system may be an appropriate response to an inadequate increase in plasma volume in gestational hypertension. Overall, the lack of irreversible changes in autonomic nervous function in hypertensive women is a good message to women with gestational hypertension considering future pregnancies.
Acknowledgement
This study was financially supported by the Kuopio University Hospital EVO 5302419.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Duvekot JJ, Peeters LL. Renal hemodynamics and volume homeostasis in pregnancy. Obstet Gyneol Surv. 1994a;49: 830–839.
- Rang S, Wolf H, Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy-associated hypertensive disorders: A review. J Hypertens. 2002;20:2111–119.
- Robson SC, Hunter S, Moore M, Dunlop W. Haemodynamic changes during the puerperium: A Doppler and M-mode echographic study. Br J Obstet Gynecol. 1987b;94: 1028–1039.
- Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. Am J Obstet Gynecol. 1994;170:849–856.
- Ekholm EMK, Erkkola RU. Autonomic cardiovascular control in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:29–36.
- Heiskanen N, Saarelainen H, Valtonen P, Lyyra-Laitinen T, Laitinen T, Vanninen E, . Blood pressure and heart rate variability analysis of orthostatic challenge in normal human pregnancies. Clin Physiol Funct Imaging. 2008;28:384–390.
- Tihtonen KM. Maternal haemodynamics in hypertensive and normotensive pregnancy. Tampere: Tampere University Press; 2006.
- Silver HM, Seebeck M, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol. 1998;179:887–893.
- Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: A longitudinal study. Obstet Gynecol. 1990;76: 1061–1069.
- Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978–984.
- Borghi C, Esposti DD, Immordino V, Cassani A, Boschi S, Bovicelli L, Ambrosioni E. Relationship of systemic hemodynamics, left ventricle structure and function, and plasma natriuretic peptide concentrations during pregnancy complicated by preeclampsia. Am J Obstet Gynecol. 2000;183: 140–147.
- San-Frutos LM, Fernandez R, Almagro J, Barbancho C, Salazar F, Perez-Medina T, Bueno B, Bajo J. Measure of hemodynamic patterns by thoracic electrical bioimpedance in normal pregnancy and in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;121:149–153.
- Eneroth-Grimfors E, Westgren M, Ericson M, Ihrman-Sandahl C, Lindblad LE. Autonomic cardiovascular control in normal and pre-eclamptic pregnancy. Acta Obstet Gynecol Scand. 1994;73:680–684.
- Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G. Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;10: 538–543.
- Laitinen T, Niskanen L, Geelen G, Länsimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004;96: 2333–2340.
- Wesseling KH, Jansen JR, Settels JJ, Schreuder JJ. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. J Appl Physiol. 1993;74:2566–2573.
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17: 354–381.
- de Boer RW, Karemaker JM, Strackee J. Relationships between short-term blood-pressure fluctuations and heart-rate variability in resting subjects. I: A spectral analysis approach. Med Biol Eng Comput. 1985;23:352–358.
- Laitinen, T, Niskanen L, Geelen G, Länsimies E, Hartikainen J. Age dependency of cardiovascular autonomic responses to head-up tilt in healthy subjects. J Appl Physiol. 2004;96: 2333–2340.
- Robbe HW, Mulder LJ, Rüddel H, Langewitz WA, Veldman JB, Mulder G, . Assessment of baroreceptor reflex sensitivity by means of spectral analysis. Hypertension. 1987;10: 538–543.
- Penttilä J, Snapir A, Kentala E, Koskenvuo J, Posti J, Scheinin M, . Estimation of cardiac output in a pharmacological trial using a simple method based on arterial blood pressure signal waveform: A comparison with pulmonary thermodilution and echocardiographic methods. Eur J Clin Pharmacol 2006;62:401–407.
- Yang J-M, Yang Y-C, Wang K-G. Central and peripheral hemodynamics in severe preeclampsia. Acta Obstet Gynecol Scand. 1996;75:120–126.
- Kaltoft N, Hobolth L, Møller S. Noninvasive measurement of cardiac output by Finometer in patients with cirrhosis. Cli Physiol Funct Imaging 2010;30:230–233.
- Laitinen T, Huopio H, Vauhkonen I, Camaro C, Hartikainen J, Laakso M, Niskanen L. Effects of euglycaemic and hypoglycaemic hyperinsulinaemia on sympathetic and parasympathetic regulation of haemodynamics in healthy subjects. Clin Sci (Lond). 2003;105:315–322.
- Sukalich S, Mingione MJ, Glantz JC. Obstetric outcomes in overweight and obese adolescents. Am J Obstet Gynecol. 2006;195, 851–855.
- Rang S, Wolf H, Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy-associated hypertensive disorder: A review. J Hypertens. 2002;20:2111–2119.
- Ekholm EM, Tahvanainen KU, Metsälä T. Heart rate and blood pressure variabilities are increased in pregnancy-induced hypertension. Am J Obstet Gynecol 1997;177: 1208–1212.
- Molino P, Veglio F, Genova GC, Melchio R, Benedetto C, Chiarolini L, . Baroreflex control of heart rate is impaired in pre-eclampsia. J Human Hypertens. 1999;13:179–183.
- Del Bene R, Barletta G, Mello G, Lazzeri C, Mecacci F, Parretti E, . Cardiovascular function in pregnancy: Effects of posture. BJOG. 2001;108:344–352.
- Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR interval oscillations in human. Circulation. 1998;98:547–555.