Abstract
Objective. To evaluate the efficacy and safety of valsartan in Taiwanese patients with essential hypertension. Methods. This 12-week multi-center, open-label, observational, post-marketing surveillance study enrolled 2046 hypertensive patients who were prescribed valsartan 80 or 160 mg as monotherapy or in combination with other antihypertensives based on clinical judgment. The primary endpoint was the incidence rate of dizziness with valsartan 160 mg monotherapy or combination therapy at Week 4. Secondary endpoints included the blood-pressure-lowering efficacy and the overall safety and tolerability of valsartan at Weeks 4 and 12. Results. The monotherapy and combination groups had comparable baseline characteristics. At Week 4, monotherapy was found non-inferior to combination for incidence rate of dizziness (monotherapy, 9.25%; combination, 10%; difference in incidence of dizziness, 0.75%; 95%CI − 0.61% to 2.12%; non-inferiority margin, −1.33%; Wald Test approach). Greater blood pressure (BP) reduction was noted at Week 12 than at Week 4. The antihypertensive effect was greater with combination therapy and the 160-mg dose. BP control (systolic <140 mmHg or diastolic <90 mmHg) was achieved in 80–90% patients. Valsartan was well tolerated; most commonly reported adverse events included dizziness, headache, constipation and cough. Conclusion. Valsartan is an effective treatment option for essential hypertension in Taiwanese patients.
Introduction
Valsartan is an orally active, non-peptide angiotensin receptor blocker (ARB) extensively used for the treatment of hypertension and heart failure worldwide for over a decade (Citation1,Citation2). The drug has been approved for the initial treatment of essential hypertension in Taiwan (2007) and USA (Citation2), and has also been included as a first-line therapeutic option in hypertensive patients by the European Society of Hypertension/European Society of Cardiology (ESH/ESC) (Citation3). Its antihypertensive action occurs because of the site specificity of valsartan, causing inhibition of the vasoconstrictor and pressor response exerted by angiotensin II; the subsequent decrease in sodium retention and aldosterone secretion is suggested to be responsible for reduction of the adverse reactions normally associated with angiotensin-converting enzyme (ACE) inhibitors (Citation4). Valsartan is used either as monotherapy or in combination with other antihypertensive agents in doses of 80, 160 and 320 mg (Citation1).
The efficacy of valsartan is known to be independent of age, sex and race, and is equivalent to that of other antihypertensive drugs like calcium antagonists, ACE inhibitors and thiazide diuretics (Citation5). Various studies have compared valsartan with placebo (Citation6) and other blood-pressure-lowering agents such as enalapril (Citation7,Citation8), amlodipine (Citation9,Citation10), losartan (Citation11), ACE inhibitors (Citation12) and beta-blockers (Citation13). The results of these studies indicate that valsartan is safe, effective and well tolerated in patients with mild to moderate hypertension.
Valsartan has a good overall safety profile, with very low incidence of side-effects, almost similar to that of placebo (Citation1,Citation3). Headache, dizziness and fatigue are frequently observed in valsartan recipients; however, dizziness is the most common reason for discontinuation of therapy (Citation4). Dizziness is also commonly observed in the general population, with an incidence rate of 3.1% and a prevalence rate of about 23% (Citation14). The incidence of dizziness with valsartan 20–160 mg is shown to be 2–3.5%, which is much lower than 5.4% reported with placebo; increase in dosage also leads to increased incidence of dizziness (Citation6). This side-effect has been attributed to the first-dose hypotensive effect; therefore, it is suggested that ARBs should be initially started in low doses and then titrated according to their hypertensive effect (Citation15). Our study assessed the incidence of dizziness associated with the higher dose of valsartan in monotherapy and in combination with other antihypertensive drugs in Taiwanese patients. We considered dizziness from a dual perspective, i.e. efficacy and safety. Although the safety and tolerability profile of valsartan is well documented in the Caucasian, African-American and Middle Eastern populations (Citation4,Citation16,Citation17), it may vary in the Asian population because of differences in body size.
The objective of this study is to evaluate the incidence rate of dizziness and the efficacy and safety of valsartan (80 and 160 mg) in monotherapy and in combination with other antihypertensive drugs (combination therapy) in the Taiwanese population.
Methods
Study design
This was a 12-week, multi-center, open-label, non-comparative, observational, post-marketing surveillance study, designed and implemented in accordance with ICH GCP guidelines and approval from the local ethics committee (Ethics Review Board). The primary objective was to evaluate the incidence rate of dizziness after 4 weeks of treatment with valsartan 160 mg as monotherapy or combination therapy. The secondary objectives included: (i) evaluation of safety and tolerability of valsartan 80 or 160 mg monotherapy or combination therapy at Week 4 and Week 12; (ii) evaluation of the blood-pressure-lowering effect of these treatments at Week 4 and Week 12; and (iii) observation of current treatment trends of hypertension, including percentage of various regimens at Week 0 and changes at Week 4 and Week 12.
Patients
Individuals aged ≥18 years and diagnosed with hypertension (sitting systolic blood pressure [SBP] ≥140 mmHg and/or diastolic blood pressure [DBP] ≥90 mmHg) were included in the study. Individuals with known hypersensitivity to valsartan or any component in the formulation; pregnant or breastfeeding women; and individuals with severe medical conditions, using any other investigational drugs at the time of enrolment, or with a history of any malignancy within the previous 5 years (except localized basal cell carcinoma of the skin) were excluded from the study. The protocol was in accordance with the ethical principles laid down in the Declaration of Helsinki, and all patients provided written informed consent.
Treatment and assessments
At the initial visit, in addition to physical examination, relevant medical history and information on concomitant medications (including antihypertensives) were recorded. Blood pressure (BP) was recorded in the sitting position in the upper arm. Based on the physician's clinical judgment, patients were prescribed valsartan 80 or 160 mg monotherapy or combination therapy. At the second visit (Week 4), BP was recorded, and any change in antihypertensive medication and adverse events (AEs) were noted. Treatment changes were determined by the physician based on BP control and tolerability. Similar assessments were made at the final visit (Week 12).
Outcome measures
The incidence rate of dizziness at Week 4 with valsartan 160 mg as monotherapy or combination therapy (primary endpoint) was assessed based on the patient's response to the standard question, “Did you feel or sense dizziness in the past 4 weeks?” The blood-pressure-lowering effect of valsartan was assessed based on the mean change from baseline in SBP and DBP at Week 4 and Week 12. An exploratory analysis was performed to compare the blood-pressure-lowering effect between the 80- and 160-mg doses. Furthermore, a subgroup analysis was conducted for patients with baseline SBP >160 and ≤160 mmHg and baseline DBP >100 and ≤100 mmHg. The BP control rate, defined as the proportion of patients with SBP <140 mmHg or DBP <90 mmHg, with valsartan 80 and 160 mg as monotherapy and combination therapy at Week 4 and Week 12 was also determined. The treatment trend was assessed based on the percentage of patients prescribed valsartan 80 or 160 mg at baseline and the percentage of patients requiring combination therapy or a switch to another monotherapy or another combination therapy at Week 4 and Week 12. The safety and tolerability of valsartan 80 or 160 mg monotherapy or combination therapy was assessed based on the incidence of AEs, serious adverse events (SAEs), and their relationship to the study drug.
Statistical analysis
Since the incidence rate of dizziness with valsartan 160 mg is 4% in Caucasians, under the non-inferiority hypothesis and with 1.33% as the clinical margin, an estimated sample size of 1336 patients would be required to reach statistical significance for the eligible population (alpha = 0.05; 1 − beta = 0.8). If the actual incidence rate of dizziness was expected to be 4.2% (5% more than reference), at least 1937 patients would be required to be enrolled in this study. The primary efficacy evaluation was conducted on the intent-to-treat (ITT) population comprising all enrolled patients who received at least one dose of valsartan and completed at least the 4-week core period. Secondary endpoints and safety were evaluated in the safety population comprising all enrolled patients who received at least one dose of valsartan during the study period.
For the primary variable, a frequency table was generated using descriptive statistics, and the incidence rate of dizziness was compared between the monotherapy and combination groups by using Pearson chi-square or Fisher's exact test. For the secondary variables, all continuous variables were summarized with descriptive statistics, and categorical variables were tabulated as frequencies and percentages. For comparison of assessments, the overall change in SBP and DBP from baseline and change in SBP and DBP by visit were analyzed by analysis of variance (ANOVA) or covariance (ANCOVA). All analyses were performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA); p < 0.05 was considered statistically significant.
Results
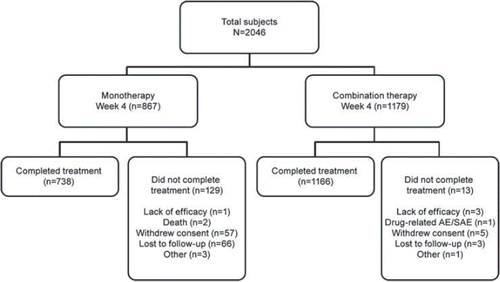
A total of 2046 patients were enrolled in the study, with 867 in monotherapy and 1179 in the combination group (). The monotherapy and combination groups were balanced in terms of demographics and baseline characteristics (). Over the study period (three visits), 53–56% patients received valsartan 80 mg and 43–44% patients received the 160-mg dose. The mean (±SD) duration of drug exposure was 81.48 ± 16.87 days in patients receiving valsartan 80 mg and 80.19 ± 18.55 days in those receiving valsartan 160 mg.
Table I. Baseline demographics and clinical characteristics of the study population.
Efficacy
At Week 4, dizziness was experienced by 27 (9.25%) and 52 (10.00%) patients receiving valsartan 160 mg in the monotherapy and combination therapy groups, respectively, in the ITT population. The difference in the incidence rate of dizziness between the monotherapy and combination therapy groups was 0.75% (95% CI − 0.61% to 2.12%). The lower margin of the confidence interval (−0.61%) was greater than the margin of the non-inferiority hypothesis (−1.33%). Thus, the monotherapy group was found to be non-inferior to the combination therapy group with respect to the incidence rate of dizziness with valsartan 160 mg at Week 4. Results of supplemental analysis in the per-protocol population were in agreement with the above results (data not shown).
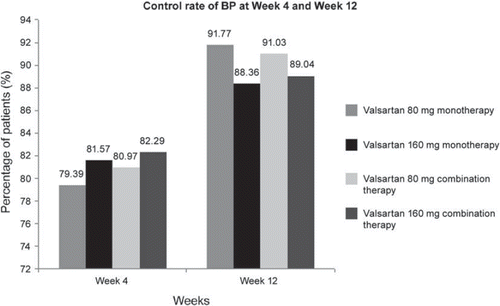
The decreases in SBP and DBP in the combination therapy group were greater than those in the monotherapy group at both Week 4 and Week 12 (). The difference between the monotherapy and combination therapy groups was statistically significant only in the case of SBP reduction at Week 12 (−18.07 ± 13.64 mmHg vs −20.37 ± 17.49 mmHg; p = 0.0293). An exploratory analysis comparing the BP reduction between the 80- and 160-mg doses in monotherapy and combination therapy showed that the reductions in both SBP and DBP were greater with the 160-mg dose than with the 80-mg dose, although statistical significance was reached only for SBP reduction (). Furthermore, the reduction in BP from baseline was greater at Week 12 than at Week 4 for both doses as well as for monotherapy and combination therapy. Subgroup analysis showed that the mean reduction in SBP and DBP in the >160/100 mmHg subgroup was almost double of that in the ≤160/100 mmHg subgroup in patients receiving valsartan 80 or 160 mg as monotherapy or combination therapy at both Week 4 and Week 12 ( and ). High BP control rates of 80% and 90% were obtained at Week 4 and Week 12, respectively, with valsartan 80- and 160-mg doses both in monotherapy and in combination therapy ().
Figure 2. (a) Mean change in systolic blood pressure (SBP) at Week 4 and Week 12 (subgroup analysis). (b) Mean change in diastolic blood pressure (DBP) at Week 4 and Week 12 (subgroup analysis).
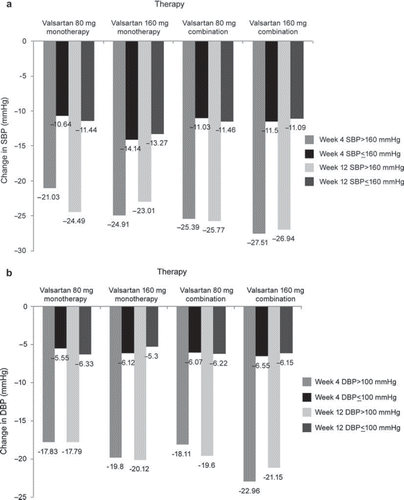
Table IIa. Overall change in blood pressure by treatment groups.a
Table IIb. Overall change in blood pressure by dose.a
Analysis of treatment trends showed that at baseline, 55.91% patients were prescribed valsartan 80 mg and 44.09% patients were prescribed valsartan 160 mg, either as monotherapy or combination therapy. At Week 4, 64.30% patients required combination therapy, 3.59% were switched to another monotherapy, and 5.42% patients were switched to another combination therapy. At Week 12, 69.72% patients required combination therapy, 7.04% were switched to another monotherapy and 4.92% patients were switched to another combination therapy.
Safety
Valsartan treatment was well tolerated; about 11% patients in both the monotherapy and combination therapy groups reported AEs. A majority of the AEs were of mild to moderate intensity, were not related to the study medication, and did not result in a change in study medication. The most frequently reported AEs (≥1%) included dizziness (monotherapy vs combination, 3.58% vs 4.33%), headache (1.04% vs 1.44%), constipation (1.04% vs 0.76%) and cough (1.04% vs 0.76%). Although at Week 4, 28.89% and 22.50% AEs in the monotherapy and combination groups, respectively, were considered probably related to the study medication, between Week 4 and Week 12, only 5.32% and 7.78% AEs were probably related to the study medication ().
Table III. Summary of adverse events (AEs).a
Two deaths were reported in the monotherapy group; both were unrelated to valsartan treatment. SAEs were noted in five patients on valsartan monotherapy and in six patients on combination therapy. None of the SAEs in the monotherapy group was suspected to be related to the study drug. In the combination group, one case of hyperkalemia with hypovolemic shock and one case of orthostatic hypotension were suspected to be related to the study drug; however, both patients recovered completely.
Discussion
Studies have shown that ARBs are generally effective and well-tolerated antihypertensive drugs (Citation3,Citation18); we evaluated the efficacy and safety of valsartan as monotherapy and combination therapy in the Taiwanese population. We assessed the occurrence of dizziness both from the general and safety viewpoints. The incidence rates of dizziness (primary endpoint) with valsartan 160 mg as monotherapy and as combination therapy at Week 4 were similar. This result suggests that the incidence of dizziness did not increase with the addition of valsartan to other antihypertensive drugs. Dizziness was reported as the most frequently occurring AE in both the groups. Although not statistically significant, subjects in the combination group had a slightly higher incidence of dizziness compared with those on monotherapy (4.33% vs 3.58%). This may be related to their underlying co-morbidity or may be because of the side-effects from multiple antihypertensive drugs. Similar observations have been made previously. The incidence of dizziness with valsartan 20–160 mg was reported to be between 2.1% and 3.4% compared with 5.4% with placebo (Citation6). A similar incidence of dizziness was reported with losartan (4%) compared with placebo (2.4%) (Citation19). Dizziness is known to be a drug-related side-effect that occurs in about 2–4% of patients taking ARBs, which is occasionally associated with a first-dose hypotensive effect (< 1% patients) (Citation2,Citation4,Citation6,Citation17,Citation20). Our study reported a low incidence of dizziness (3.5–4.3%) in the Taiwanese population.
Greater BP reduction was observed at Week 12 than at Week 4. Furthermore, the mean SBP reduction at Week 12 was significantly greater in the combination group than in the monotherapy group. This could be attributed to an additional antihypertensive effect from the other drug (calcium-channel blocker, diuretic, etc.) in the combination therapy. These results are in accordance with those reported by Lacourcière et al. (Citation21), wherein significant reductions in SBP and DBP were observed with valsartan in combination therapy compared with monotherapy. A study by Calhoun et al. (Citation22) also showed that mean reductions in BP were significantly greater with valsartan in combination therapy than as monotherapy at Week 4, with further reductions seen at Week 6. Similar results were achieved by Mallion et al. (Citation23) at the end of 12 weeks with valsartan combination therapy vs monotherapy.
The mean age of our sample population was approximately 62 years. Previous studies have shown that the reductions in SBP and DBP were clinically and statistically significant, with SBP changes generally being higher in elderly patients than in younger subjects (Citation10,Citation23,Citation24). The study also compared the efficacy of the 80- and 160-mg doses of valsartan for lowering BP at Weeks 4 and 12. The reduction in SBP from baseline was significantly greater in the 160 mg group than in the 80 mg group at both time points. Similar results have been reported by Black et al. (Citation2), with valsartan exhibiting dose-dependent efficacy in reducing BP over the once-daily dose range of 80–320 mg. Our results are also compatible with those of Pool et al. (Citation25), who showed that valsartan 80, 160 and 320 mg administered once a day produces dose-related decreases in SBP and DBP, with a difference from placebo of approximately 6–9/3–5 mmHg at 80–160 mg and 9/6 mmHg at 320 mg.
The proportion of patients with SBP < 140 mmHg and DBP < 90 mmHg at Week 12 (90%) was greater than that at Week 4 (80%). The 2002 Taiwanese Survey on Hypertension, Hyperglycemia, and Hyperlipidemia (TwSHHH) study reported a control rate of 24.3% in hypertensive patients (Citation26). Although the TwSHHH study defined “controlled hypertension” similar to that in our study, the results should be interpreted with caution because of differences in study design and settings between both the studies. Valsartan showed twice the amount of reduction in BP in the subgroup of patients with higher baseline levels of SBP and DBP (>160/100 mmHg) than those with lower baseline levels in both treatment groups. Similar results have been observed in previous studies. Greater BP reduction has been reported in patients with higher baseline SBP and DBP levels (> 160/100 mmHg) with valsartan therapy compared with other antihypertensive drugs (Citation13,Citation27,Citation28,Citation29). Valsartan can prove to be a valuable option in these patients either as monotherapy with the 160-mg dose or as combination therapy.
The overall safety profiles of the two groups were found to be similar. Valsartan was well tolerated with a low incidence of AEs and SAEs. Most AEs were of mild to moderate intensity and resolved. The safety and tolerability results of valsartan are known to be independent of dose and duration of treatment and are consistent regardless of age, gender and ethnic group at doses up to 320 mg/day (Citation2). The main advantage of ARBs over ACE inhibitors is the reduced incidence of cough and angioedema (Citation2,Citation30,Citation31). Dry cough was previously reported in 7.9% patients on ACE inhibitors compared with only 2.6% patients receiving valsartan therapy (Citation2). This is agreement with our results, where cough was reported in only 1.04% and 0.76% patients in the monotherapy and combination groups, respectively.
Various trends in treatment changes at Weeks 4 and 12 were observed in this study. At Week 4, 64.30% patients required combination therapy, and 3.59% patients were switched from the 80-mg dose to the 160-mg dose as monotherapy. At Week 12, the percentage of patients requiring combination therapy increased to 69.72%, and those that switched to another monotherapy increased to 7.04%. This switch could have been because the desired results were not achieved with the existing doses. Similar observations have been made with another ARB, candesartan, which showed better results when used as an add-on (combination therapy) with other antihypertensive drugs (Citation32). This finding seems to be in line with the ESC guidelines that suggest that effective BP control can only be achieved by a combination of at least two antihypertensive drugs (Citation33).
The results of our study have limitations that are usually associated with observational studies, including bias in the assignment of treatment groups by the physician. Another source of bias is the absence of a control group. However, our results are relevant and important because of the large number of patients selected from the general population with few exclusion criteria and provide real-life evidence of the efficacy and safety of valsartan in Taiwanese patients with essential hypertension.
In conclusion, valsartan can be an effective treatment option for patients with essential hypertension in Taiwan.
Acknowledgments
We would like to thank the investigators Chang Hsun-Hsieh, Chang-Hung Hsu and Jun-Ting Liu (Tri-Service General Hospital); Yu-Cheng Hsu (Lotung Poi-Ai Hospital); Shih-Te Tu, Shi-Li Su, Dong-Hwa Tsai, Shang-Ren Hsu and Shu-Yi Wang (Changhua Christian Hospital); Ku-Chou Chang, Teng-Yeow Tan, Wen-Neng Chang, Cheng-Hsien Lu, Shang-Der Chen, Min-Yu Lan, Hung-Sheng Lin and Wei-His Chen (Kaohsiung Chang Gung Memorial Hospital); Shi-Sheng Chang (China Medical University Hospital); Ming-Cheng Peng, Chung-Lieh Hung, Chih-Hsuan Yen and Ping-Ying Lee (Mackay Memorial Hospital); Wei-Kung Tseng, Chin-Feng Hsuan, Thung-Lip Lee and Ya-Feng Pan (E-Da Hospital); Shih-Kai Lin, Chung-Peng Liu, Pei-Leun Kang, Hwong-Ru Hwang, Chi-Cheng Lai, Feng-Yu Kuo, Chin-Chang Cheng and Den-Ko Wu (Kaohsiung Veterans General Hospital); Pei-Chieh Fong (Novartis Pharmaceuticals Ltd., Taiwan) for their valuable contributions to this project. We would like to acknowledge the services of Jo Chen (Qualitix Clinical Research Co. Ltd.) for statistical assistance. We also thank the study participants and their families for their personal time and commitment to this project. Supportive medical writing services were offered by Cactus Communications Pvt. Ltd. and paid for by Novartis Taiwan.
Disclosure
The current study was sponsored by Novartis for post-marketing surveillance of valsartan with regard to its efficacy, tolerability and safety profiles in Asia-Pacific patients. Jackson Wang is an employee of Novartis and is therefore eligible for Novartis stock and stock options. All investigators have received investigator fees from Novartis. None of the other authors has any conflict of interest to declare.
References
- Kjeldsen SE, Brunner HR, McInnes GT, Stolt P. Valsartan in the treatment of hypertension. Aging Health. 2005;1:27–36.
- Black H, Bailey J, Zappe D, Samuel R. Valsartan: More than a decade of experience. Drugs. 2009;69:2393–2414.
- Verdecchia P, Angeli F, Mazzotta G, Ambrosio G, Reboldi G. Angiotensin receptor blockers in hypertension. New insights from Japan. Hypertens Res. 2010;33:394–397.
- Biswas PN, Wilton LV, Shakir SW. The safety of valsartan: Results of a postmarketing surveillance study on 12881 patients in England. J Hum Hypertens. 2002;16:795–803.
- McInnes GT. Clinical advantage of valsartan. Cardiology. 1999;91:14–18.
- Oparil S, Dyke S, Harris F, Kief J, James D, Hester A, . The efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertension. Clin Ther. 1996;18:797–810.
- Holwerda NJ, Fogari R, Angeli P, Porcellati C, Hereng C, Oddou-Stock P, . Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: Efficacy and safety compared with placebo and enalapril. J Hypertens. 1996;14:1147–1151.
- Fogari R, Mugellini A, Zoppi A, Marasi G, Pasotti C, Poletti L, . Effects of valsartan compared with enalapril on blood pressure and cognitive function in elderly patients with essential hypertension. Eur J Clin Pharmacol. 2004;59:863–868.
- Corea L, Cardoni O, Fogari R, Innocenti P, Porcellati C, Provvidenza M, . Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: A comparative study of the efficacy and safety against amlodipine. Clin Pharmacol Ther. 1996;60:341–346.
- Malacco E, Varì N, Capuano V, Spagnuolo V, Borgnino C, Palatini P, . A randomized, double-blind, active-controlled, parallel-group comparison of valsartan and amlodipine in the treatment of isolated systolic hypertension in elderly patients: The Val-Syst study. Clin Ther. 2003;25:2765–2780.
- Hedner T, Oparil S, Rasmussen K, Rapelli A, Gatlin M, Kobi P, . A comparison of the angiotensin II antagonists valsartan and losartan in the treatment of essential hypertension. Am J Hypertens. 1999;12:414–417.
- Black HR, Graff A, Shute D, Stoltz R, Ruff D, Levine J, . Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: Efficacy, tolerability and safety compared to an angiotensin-converting enzyme inhibitor, lisinopril. J Hum Hypertens. 1997;11:483–489.
- Cifkova R, Peleska J, Hradec J, Rosolová H, Pintérová E, Zeman K, . Valsartan and atenolol in patients with severe essential hypertension. J Hum Hypertens. 1998;12:563–567.
- Neuhauser H, Radtke A, von Brevern M, Lezius F, Feldmann M, Lempert T. Burden of dizziness and vertigo in the community. Arch Intern Med. 2008;168:2118–2124.
- Kirk JK. Angiotensin-II receptor antagonists: Their place in therapy. Am Fam Physician. 1999;59:3140–3148.
- Weir MR, Ferdinand KC, Flack JM, Jamerson KA, Daley W, Zelenkofske S. A non-inferiority comparison of valsartan/hydrochlorothiazide combination versus amlodipine in black hypertensives. Hypertension. 2005;46:508–513.
- Nouh MS, Halim SA. Efficacy and tolerability of valsartan in patients with mild to moderate essential hypertension. Saudi Med J. 2002;23:521–525.
- Weir MR. Angiotensin II receptor blockers: The importance of dose in cardiovascular and renal risk reduction. J Clin Hypertens (Greenwich). 2004;6:315–323.
- Weber M. Clinical safety and tolerability of losartan. Clin Ther. 1997;19:604–616.
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675.
- Lacourcière Y, Poirier L, Hebert D, Assouline L, Stolt P, Rehel B, . Antihypertensive efficacy and tolerability of two fixed-dose combinations of valsartan and hydrochlorothiazide compared with valsartan monotherapy in patients with stage 2 or 3 systolic hypertension: An 8-week, randomized, double-blind, parallel-group trial. Clin Ther. 2005;27:1013–1021.
- Calhoun DA, Glazer RD, Pettyjohn FS, Coenen PD, Zhao Y, Grosso A. Efficacy and tolerability of combination therapy with valsartan/hydrochlorothiazide in the initial treatment of severe hypertension. Curr Med Res Opin. 2008;24:2303–2311.
- Mallion JM, Carretta R, Trenkwalder P, Martinez JF, Tykarski A, Teitelbaum I, . Valsartan/hydrochlorothiazide is effective in hypertensive patients inadequately controlled by valsartan monotherapy. Blood Press Suppl. 2003;1:36–43.
- Neutel JM, Bedigian MP. Efficacy of valsartan in patients aged 65 years with systolic hypertension. Clin Ther. 2000;22:961–969.
- Pool J, Oparil S, Hedner T, Glazer R, Oddou-Stock P, Hester A. Dose-responsive antihypertensive efficacy of valsartan, a new angiotensin II-receptor blocker. Clin Ther. 1998;20:1106–1114.
- Su TC, Bai CH, Chang HY, You SL, Chien KL, Chen MF, Chen HJ, . Evidence for improved control of hypertension in Taiwan:. 1993–2002. J Hypertens. 2008;26:600–606.
- Nixon RM, Müller E, Lowy A, Falvey H. Valsartan vs other angiotensin II receptor blockers in the treatment of hypertension: A meta-analytical approach. Int J Clin Pract. 2009;63:766–775.
- Ruilope LM, Malacco E, Khder Y, Kandra A, Bönner G, Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: The VAST study. Clin Ther. 2005;27:578–587.
- Fogari R, Zoppi A, Carretta R, Veglio F, Salvetti A, Italian Collaborative Study Group. Effect of indomethacin on the antihypertensive efficacy of valsartan and lisinopril: A multicentre study. J Hypertens. 2002;20:1007–1014.
- Lacourcière Y, Lefebvre J, Nakhle G, Faison EP, Snavely DB, Nelson EB. Association between cough and angiotensin converting enzyme inhibitors versus angiotensin II antagonists: The design of a prospective, controlled study. J Hypertens Suppl. 1994;12:S49–53.
- Pylypchuk GB. ACE inhibitor versus angiotensin II blocker-induced cough and angioedema. Ann Pharmacother. 1998;32:1060–1066.
- Weir MR, Weber MA, Neutel JM, Vendetti J, Michelson EL, Wang RY. Efficacy of candesartan cilexetil as add-on therapy in hypertensive patients uncontrolled on background therapy: A clinical experience trial. Am J Hypertens. 2001;14:567–572.
- Mancia G, Laurent S, Agabati-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, . Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158.