Abstract
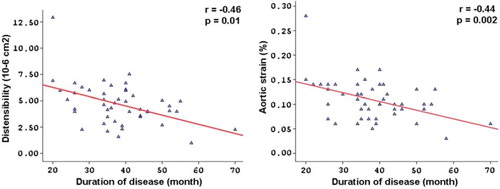
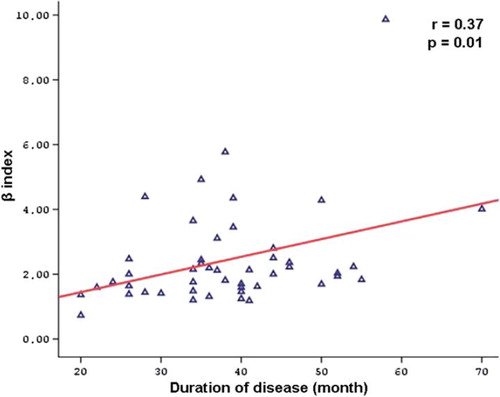
Background. Sarcoidosis is an inflammatory granulomatous disease of unknown etiology that involves multiple organ systems. Many studies have shown a strong relationship between inflammation and atherosclerosis. The aim of this study is to investigate the relationship between elastic properties of the aorta and the duration of the disease in patients with sarcoidosis. Method. The study population included 52 patients with sarcoidosis (22 men, mean age = 42.7 ± 10.7 years, and mean disease duration = 38.8 ± 10.8 months) and 50 healthy control subjects (18 men, and mean age = 42.0 ± 8.0 years). Aortic stiffness (β) index, aortic strain (AoS) and aortic distensibility (AoD) were calculated from the aortic diameters measured by transthoracic echocardiography and blood pressure obtained by sphygmomanometer. Cardiac functions were determined by using routine echocardiographic evaluation consist of standard two-dimensional and conventional Doppler and tissue Doppler imaging. Results. The conventional echocardiographic parameters were similar between patients and controls. There were significant differences between the control and the patient groups in β index (1.63 ± 0.55 vs 2.44 ± 1.54, p = 0.001), AoS (15.61 ± 5.69 vs 10.93 ± 4.11%, p < 0.001) and AoD (6.35 ± 2.64 vs 4.66 ± 1.98, 10 −6 cm2/dyn, p = 0.001). There were statistically significant negative correlations between the disease duration and AoD (r = −0.46, p = 0.01) and AoS (r= −0.44, p = 0.002), whereas there was a positive correlation between the disease duration and β index (r = 0.37, p = 0.01). In multivariate analysis, disease duration was significantly related with AoD, AoS and β index (respectively, RR = 3.28, p = 0.002; RR = 3.03, p = 0.004; RR = 2.39, p = 0.02). Conclusion. We observed that elastic properties of the aorta alter in patients with sarcoidosis. We also have demonstrated a statistically significant correlation between aortic elastic properties and the disease duration.
Introduction
Sarcoidosis is an inflammatory granulomatous disease of unknown etiology that involves multiple organ systems, including the lungs, heart, eyes, liver and skin (Citation1). Cardiac involvement has been reported at an incidence of about 25% of sarcoidosis cases in US and nearly 60% of Japanese patients with sarcoidosis in autopsy studies (Citation2). Cardiac involvement in sarcoidosis includes heart blocks, congestive heart failure, diastolic dysfunction, left ventricular (LV) wall motion abnormalities, pericardial and valvular heart disease, ventricular arrhythmias and sudden cardiac death (Citation1–3).
Non-invasive assessment of the aortic elastic properties can be done by evaluation of pulsatile changes in ascending aorta with transthoracic echocardiography. Previous studies have shown that non-invasive estimation of aortic distensibility (AoD) by evaluation of aortic dimensions with echocardiography and blood pressure data achieve almost the same accuracy as invasive methods do (Citation4). Aortic elastic properties reflect aortic stiffness, which is related to subclinical atherosclerosis and predicts cardiovascular morbidity and mortality (Citation5). Aortic stiffness has been shown to be in association with a variety chronic inflammatory disorders, including rheumatoid arthritis, psoriasis, familial Mediterranean fever and chronic graft-versus-host disease (Citation6–9).
There is only one study present to investigate the effects of sarcoidosis on AoD but there is no study yet to evaluate aortic strain (AoS) and aortic stiffness index (β) in patient with sarcoidosis (Citation10). In the present study, we aimed to investigate the relationship between aortic elastic properties (β index, AoS and AoD) and the duration of the disease in patients with sarcoidosis.
Method
Study population
The study population included 52 consecutive patients with sarcoidosis who were referred from our Chest Disease Department (22 men; mean age, 42.7 ± 10.7 years, and mean disease duration =38.8 ± 10.8 months) and 45 healthy subjects as controls (18 men; mean age = 42.0 ± 8.0 years). The diagnosis of sarcoidosis was established according to the American Thoracic Society recommendations (Citation11). Age, gender, body mass index (BMI) and biochemical measurements were recorded. The demographic characteristics and clinical features of the patients and the controls are given in .
Table I. Demographic and clinical futures of the patients and the controls.
The control subjects had no cardiovascular or any other organ system disease and with normal physical examination, chest roentgenogram, electrocardiogram, and two-dimensional and Doppler echocardiogram. None of the patients had hypertension, renal failure, diabetes mellitus, LV ejection fraction lower than 50%, severe valvular regurgitation and moderate or severe valvular stenosis, coronary artery disease, chronic obstructive pulmonary disease, and atrial fibrillation. The patients with poor echocardiographic image quality were also excluded. This study complied with the Declaration of Helsinki, was approved by the Ethics Committee and the institutional review board of Erciyes University Medical School, and informed consent was obtained from each patient.
Echocardiography
All patients underwent complete transthoracic echocardiographic studies including two-dimensional, color flow and spectral Doppler, as well as TDI with a GE-Vingmed Vivid 7 system (GE-Vingmed Ultrasound AS, Horten, Norway) using a 2.5-MHz transducer. Echocardiographic measurements were taken with patients in the left lateral decubitus position using standard parasternal long- and short-axis and apical views. At least three consecutive beats in sinus rhythm were recorded at simultaneous electrocardiography monitor of echocardiography and the average values were taken. All measurements were performed according to published criteria of the American Society of Echocardiography (Citation12). All echocardiographic measurements were carried out by two experienced cardiologists who were unaware of the clinical data.
Measurement of elastic properties of the aorta
Aortic stiffness was determined non-invasively based on the relationship between changes in aortic diameter and pressure with each cardiac pulse (Citation8). Ascending aorta was recorded at a level 3 cm above the aortic valve in the M-mode tracing guided by the two-dimensional echocardiography in the parasternal long-axis view. Systolic diameter (SD) was measured as the maximal anterior motion of the aorta and diastolic diameter (DD) at the peak of the QRS complex of the simultaneously recorded electrocardiogram. Five consecutive cardiac beats were measured routinely and average of the values was recorded. Blood pressure was measured at the brachial level with an external sphygmomanometer.
The following aortic elastic indices were calculated (Citation13):
AoS=(SD−DD)/DD
β index = ln (SBP/DBP)/[(SD − DD)/DD]
where SBP and DBP are the systolic and diastolic blood pressures, and “ln” is the natural logarithm.
AoD = 2 × (SD − DD)/[(SBP − DBP) × DD] × 10−6 cm2/dyn
Intraobserver and interobserver variability for the systolic and diastolic dimensions ranged from 4.1% to 5.4%.
Pulmonary evaluation
Patients with sarcoidosis underwent posteroanterior chest radiography to determine disease stage using standard radiographic staging for the disease according to the Scadding criteria (Citation14). Stage 0 describes no visible intrathoracic findings. Stage I is bilateral hilar lymphadenopathy (BHL) with normal lung parenchyma. Stage II is BHL and parenchymal infiltration. Stage III is bilateral infiltration without BHL and stage IV is pulmonary fibrosis/fibrocystic parenchymal involvement (honeycombing, hilar retraction, bullae, cysts and emphysema).
Statistical analysis
Continuous variables were given as mean ± SD; categorical variables were defined as percentage. An independent-sample t-test was used to compare the study variables between sarcoidosis patients and control subjects. Correlation analyses were performed using the Pearson coefficient of correlation. To assess the relationship between disease duration and age with aortic elastic properties, multivariate logistic regression analysis was performed, and results are shown as an odds ratio (OR) with 95% confidence intervals (CIs). A probability value of p < 0.05 was considered significant, and two-tailed p-values were used for all statistics. All statistical analyses were carried out using statistical software (SPSS, version 13.0 for Windows; SPSS, Chicago, IL).
Results
Characteristics of the study groups are shown in . According to the basic clinical and demographic characteristics, both groups were similar with regard to age, BMI, fasting glucose and cholesterol levels. All subjects were normotensives and any significant difference in systolic or diastolic blood pressures or heart rate was not observed between the two groups. Systolic pulmonary arterial pressure and right ventricle Tei index were significantly higher in patients with sarcoidosis compared with healthy controls (29.3 ± 5.5 vs 25.2 ± 5.6 mmHg, p = 0.001 and 0.47 ± 0.13 vs 0.38 ± 0.08, p = 0.008). Clinical features of sarcoidosis are also listed in .
Comparison of the baseline echocardiographic values among sarcoidosis patients and the control subjects is summarized in . There was no difference between the two groups regarding to LV diameters, ejection fraction and LV diastolic filling parameters (E, A, E/A, E-Dec). On the other hand, IVRT values were higher in the patients with sarcoidosis than the controls (113.3 ± 16.3 vs 91.8 ± 19.4 ms, p = 0.001). Comparison of the tissue Doppler parameters and myocardial performance index values among sarcoidosis patients and the control subjects are also listed in .
Table II. Comparison of echocardiographic measures and aortic elastic properties of patients and controls.
Aortic elasticity parameters are also shown in . Pulse pressure and aortic diastolic diameter were increased in the sarcoidosis patients compared with control subjects. Furthermore, AoS (10.93 ± 4.11 vs 15.61 ± 5.69%, p < 0.001) and AoD were significantly decreased in the patient group (4.66 ± 1.98 vs 6.35 ± 2.64 10 −6 cm2/dyn, p = 0.001). On the other hand, β index was increased in the sarcoidosis group compared with control subjects (2.44 ± 1.54 vs 1.63 ± 0.55, p = 0.001). There was no correlation between the aortic elastic properties and the LV function parameters. There were significant negative correlations between the disease duration and AoD (r = − 0.46, p = 0.01), AoS (r = − 0.44, p = 0.002) and aortic diameter change (r = − 0.36, p = 0.01) (). On the other hand, there was a remarkably positive correlation between the disease duration and β index (r = 0.37, p = 0.01) (). In multivariate analysis, disease duration was significantly related with AoD, AoS and β index (respectively, RR = 3.28, p = 0.002; RR = 3.03, p = 0.004; RR = 2.39, p = 0.02) ().
Table III. Multivariate analysis: the relationship between the disease duration and age with aortic elastic properties of the study subjects.
Discussion
In this study, we evaluated some factors related to aortic elastic properties in patients with sarcoidosis. We demonstrated that aortic elastic properties in patients with sarcoidosis are impaired in comparison with healthy control subjects and there was a significant correlation between elastic properties of the aorta and the disease duration. We also have shown that both the systolic pulmonary arterial pressure and the right ventricular Tei index were significantly higher in patients with sarcoidosis than in healthy controls.
Sarcoidosis is a systemic granulomatous disease that involves nearly every organ of the body (Citation1–3). Clinical evidence of myocardial involvement is present in about 5% of patients with sarcoidosis. Myocardial involvement is usually associated with poor prognosis because of the development of fatal arrhythmias, atrioventricular conduction disorder or refractory congestive heart failure (Citation15). Unfortunately, diagnosis of cardiac sarcoidosis is usually difficult and those patients need more aggressive therapy (corticosteroid, immunosuppressive and immune modulator agents). For the diagnosis of cardiac involvement in a patient with sarcoidosis, endomyocardial biopsy, electrocardiography, echocardiography, myocardial perfusion scintigraphy, and 24-h Holter recordings are used (Citation16,Citation17). However, these strategies have limited sensitivity and there is no specific diagnostic test for cardiac sarcoidosis yet.
Sarcoidosis can affect small to large caliber vessels by developing granulomatous angiitis and microangiopathy, but there are limited reports in the literature to show impaired elastic properties of the aorta in patients with sarcoidosis (Citation10). Aortic elastic properties (β index, AoS and AoD), which were calculated from pulsatile changes in ascending aorta, are practically used for measuring the large arterial stiffness (Citation18). Several reports have shown that large arterial stiffness is an independent predictor of cardiovascular morbidity and mortality (Citation6,Citation7,Citation16). Recent studies have shown very good correlation between the aortic stiffness indices calculated from aortic root and the indices derived from aortography and pulse wave analysis methods (Citation19).
Previous studies have reported that several diseases can lead to increased aortic stiffness such as arterial hypertension, coronary artery disease, congestive heart failure, diabetes mellitus, chronic renal failure, Takayasu arteritis, Behçet's disease and systemic sclerosis (Citation5,Citation20–23). In a recent study, Moyssakis et al. (Citation10) have reported that AoD is significantly lower in the patients with sarcoidosis than the healthy controls and there is no significant relationship found between AoD and the disease duration. In our study, we have demonstrated a similar result that AoD significantly decreases in the patients with sarcoidosis; however, we observed a significant negative correlation between the disease duration and AoD. We have also shown that other aortic elastic properties such as β index and AoS are impaired in sarcoidosis patients compared with healthy controls and there is a significant correlation between the elastic parameters and the disease duration. It is well known that with aging aortic elasticity is impaired and it is associated with atherosclerosis and stroke. So, in our study, we excluded subjects with coronary artery disease, stroke, diabetes mellitus, hypertension and current smoking to avoid the negative effects of these variables on the aortic elasticity. In addition, both the control group and subjects with sarcoidosis had almost similar atherosclerotic risk factors such as age, systolic and diastolic blood pressure, and fasting glucose and cholesterol levels.
Impaired function and dilation of the aorta in the patients with sarcoidosis might be related to vasculitis, which affects medium-sized and large vessels (Citation24,Citation25). Elevated circulating levels of proinflammatory and inflammatory mediators such as tumor necrosis factor alpha (TNF-α), interleukin-2 (IL-2), IL-1, IL-6, IL-8, IL-10, IL-12 and IL-13, may encourage the degradation of collagen and elastin content of the aortic intima and may, thus, contribute to impaired function and dilation of the aorta (Citation26–30). The inflammatory response in sarcoidosis is characterized by the accumulation of activated T cells and macrophages at sites of ongoing inflammation and these cells spontaneously release interferon-gamma (IFN-gamma) and IL-2. Furthermore, there is an increased release of cytokines such as IL-1, IL-6, IL-8, IL-12, IL-15, IL-16, TNF-α and growth factors. Most of these cytokines favor granuloma formation and organ damage (Citation14). In the present study, we have demonstrated that there is a correlation between the duration of the disease and the impairment of elastic properties of the aortic wall. During the course of the disease, sustained exposure of the aortic wall to increased circulating cytokines may cause significant endothelial dysfunction. This mechanism may partly explain the relation between the abnormal aortic function indexes and the duration of the disease observed in our study.
Study limitations
The major limitation of our study is the design, which is cross-sectional. Pulse wave velocity (PWV), detec-ted by invasive or non-invasive methods, has been generally used to assess the large arterial stiffness, which, however, does not provide a direct measurement of the elastic properties of the aortic wall at different sites. In the present investigation, PWV was not used because the measurement of pulse velocity requires extra equipment. In this respect, to use the data obtained from pulsatile changes of ascending aorta with a standard echocardiography and pulse pressure with an ordinary sphygmomanometer is more simple and practical for the assessment of aortic elasticity. Several reports have also suggested that there is a good correlation between aortic root parameters and pulse wave analysis methods (Citation13). Another limitation of our study was that we could not detect the serum levels of inflammatory cytokines such as TNF-α, IL-1 or IL-6 in our study population. Anti-inflammatory therapies, such as corticosteroids have been shown to reduce arterial stiffness in patients with chronic inflammatory conditions. Some of our patients with sarcoidosis (23%) are treated with corticosteroids and unfortunately, we could not reach the data before and after initiation of corticosteroid therapy in order to see whether this treatment has affected the aortic elasticity. For these reasons, long-term follow-up and large-scale prospective studies are required.
In conclusion, aortic elastic properties, such as β index, AoS and AoD are altered in patients with sarcoidosis. We have also demonstrated that there is a significant correlation between aortic elastic properties and the disease duration. These results have indicated that impaired aortic elasticity may be related with the inflammation process of sarcoidosis. Non-invasive measurement of aortic elastic properties may provide an easy, cheap and practical way to follow up the progression of sarcoidosis.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Reich JM. What is sarcoidosis? Chest. 2003;124:367–171.
- Habersberger J, Manins V, Taylor AJ. Cardiac sarcoidosis. Intern Med J. 2008;38:270–277.
- Ardic I, Kaya MG, Yarlioglues M, Dogdu O, Buyukoglan H, Kalay N, . Impaired heart rate recovery index in patients with sarcoidosis. Chest. 2011;139:60–68.
- Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90:37–43.
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, . Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241.
- Tavil Y, Oztürk MA, Ureten K, Sen N, Kaya MG, Cemri M, . Assessment of aortic wall stiffness in patients with familial Mediterranean fever. Joint Bone Spine. 2008;75:280–285.
- Mäki-Petäjä KM, Booth AD, Hall FC, Wallace SM, Brown J, McEniery CM, . Ezetimibe and simvastatin reduce inflammation, disease activity, and aortic stiffness and improve endothelial function in rheumatoid arthritis. J Am Coll Cardiol 2007;50:852–858.
- Ardic I, Kaya MG, Yarlioglues M, Karadag Z, Dogan A, Yildiz H, . Impaired aortic elastic properties in normotensive patients with psoriasis. Blood Press. 2010;19:351–358.
- Dogdu O, Kaya MG, Yarlioglues M, Dogan A, Ardic I, Elcik D, . Impaired aortic elastic properties in patients with chronic graft-versus-host disease. Echocardiography 2011;28: 1011–1018.
- Moyssakis I, Gialafos E, Tentolouris N, Floudas CS, Papaioannou TG, Kostopoulos Ch, . Impaired aortic elastic properties in patients with systemic sarcoidosis. Eur J Clin Invest. 2008;38:82–89.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755.
- Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184.
- Stefanadis C, Stratos C, Boudoulas H, Kourouklis C, Toutouzas P. Distensibility of the ascending aorta: Comparison of invasive and non-invasive techniques in healthy men and in men with arterial disease. Eur Heart J. 1990;11:990–996.
- Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, . ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173.
- Sekiguchi M, Yazaki Y, Isobe M, Hiroe M. Cardiac sarcoidosis: Diagnostic, prognostic, and therapeutic considerations. Cardiovasc Drugs Ther. 1996;10:495–510.
- Homsi M, Alsayed L, Safadi B, Mahenthiran J, Das MK. Fragmented QRS complexes on 12-lead ECG: A marker of cardiac sarcoidosis as detected by gadolinium cardiac magnetic resonance imaging. Ann Noninvasive Electrocardiol. 2009;14:319–326.
- Uyarel H, Uslu N, Okmen E, Tartan Z, Kasikcioglu H, Dayi SU, . QT dispersion in sarcoidosis. Chest. 2005;128: 2619–2625.
- Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, . Cardiac cycle dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802.
- O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45:652–658.
- Ng WF, Fantin F, Ng C, Dockery F, Schiff R, Davies KA, . Takayasu's arteritis: A cause of prolonged arterial stiffness. Rheumatology (Oxford). 2006;45:741–745.
- Tunc SE, Dogan A, Gedikli O, Arslan C, Sahin M. Assessment of aortic stiffness and ventricular diastolic functions in patients with Behçet's disease. Rheumatol Int. 2005;25:447–451.
- Timár O, Soltész P, Szamosi S, Dér H, Szántó S, Szekanecz Z, . Increased arterial stiffness as the marker of vascular involvement in systemic sclerosis. J Rheumatol. 2008;35: 1329–1333.
- Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: A randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512.
- Maksimowicz-McKinnon K, Hoffman GS. Large-vessel vasculitis. Semin Respir Crit Care Med. 2004;25:569–579.
- Fernandes SR, Singsen BH, Hoffman GS. Sarcoidosis and systemic vasculitis. Semin Arthritis Rheum. 2000;30:33–46.
- Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: Who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis. 2008;25:76–89.
- Fireman E, Gilburd D, Marmor S. Angiogenic cytokines in induced sputum of patients with sarcoidosis. Respirology. 2009;14:117–123.
- Mroz RM, Korniluk M, Stasiak-Barmuta A, Chyczewska E. Increased levels of interleukin-12 and interleukin-18 in bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. J Physiol Pharmacol. 2008;59:507–513.
- Boots AW, Drent M, Swennen EL, Moonen HJ, Bast A, Haenen GR. Antioxidant status associated with inflammation in sarcoidosis: A potential role for antioxidants. Respir Med. 2009;103:364–372.
- Katsuda S, Okada Y, Okada Y, Imai K, Nakanishi I. Matrix metalloproteinase 9 (92-kd gelatinase/type IV collagenase) can degrade arterial elastin. Am J Pathol. 1994;144:1208–1218.