Abstract
Background. We aimed to determine the status of the autonomic nervous system in patients with autosomal-dominant polycystic kidney disease (ADPKD) who were normotensive and had normal renal function. Methods. A total of 28 normotensive ADPKD patients with normal renal function and 30 healthy control subjects consented to participate in the study. Heart rate recovery (HRR) indices were defined as the reduction in heart rate from the rate at peak exercise to the rate at the 1st, 2nd, 3rd and 5th minutes after the cessation of the exercise stress test; these results were indicated HRR1, HRR2, HRR3 and HRR5, respectively. Results. The 1st- and 2nd-minute HRR indices of patients with ADPKD were significantly lower than those of the healthy control group (27.1±7.9 vs 32.0±7.9; p=0.023 and 46.9±11.5 vs 53.0±9.0; p=0.029, respectively). Similarly, HRR indices after the 3rd and 5th minutes of the recovery period were significantly lower in patients with ADPKD when compared with indices in the control group (56.7±12.0 vs 65.1±11.2; p=0.008 and 62.5±13.8 vs 76.6±15.5; p =0.001, respectively). Conclusion. Impaired HRR index is associated with normotensive early-stage ADPKD patients. Increased renal ischemia and activation of the renin–angiotensin–aldosterone system (RAAS) may contribute to impairment in the autonomic nervous system in these patients before the development of hypertension. Even if ADPKD patients are normotensive, there appears to be an association with autonomic dysfunction and polycystic kidney disease.
Introduction
Reflex and usual control of heart rate is dependent upon the balance of vagal and sympathetic input; disruption of this balance and the occurrence of impairment in this regulation is related to significantly increased cardiovascular mortality (Citation1). Autonomic function can be evaluated via analysis of systolic blood pressure (SBP) variability, heart rate variability (HRV) and recovery index (HRRI) (Citation2,Citation3). Impairment in heart rate recovery (HRR) index that is present with abnormal autonomic control of the heart and vasculature is a strong predictor of subsequent cardiovascular events, and enhanced sympathetic activity is associated with the development of arrhythmias, left ventricular (LV) hypertrophy (LVH) and all-cause mortality (Citation1,Citation4).
Autosomal-dominant polycystic kidney disease (ADPKD) is a systemic hereditary disorder characterized by the growth of renal cysts, which become symptomatic during the fourth and fifth decades of life. Several studies have recently reported that there was an important link between sympathetic hyperactivity and cardiovascular morbidity in patients with ADPKD (Citation5,Citation6). Although there are few reports evaluating cardiac autonomic function in patients with ADPKD, this relation has been seen clearly and resulted in progression to dialysis (Citation7). Autonomic condition via HRRI has not been sufficiently evaluated in normotensive ADPKD patients who have normal renal function.
Using the HRR index method in this study, we aimed to determine the status of the autonomic nervous system in patients with ADPKD who are normotensive and have normal renal function.
Methods
Study protocol and parameters
The study participants were selected from the patients in the Turkish Society of Nephrology Polycystic Kidney Disease Working Group Registry, which was initiated on 30 March 2002. The diagnosis of ADPKD was confirmed by the positive family history and the presence of five or more renal cysts on renal ultrasound distributed between both kidneys. Demographic characteristics (e.g. gender, age, education status and smoking history), renal manifestations (e.g. hematuria, urinary system infection, urinary tract stones and renal replacement therapy) and cardiovascular manifestations (e.g. hypertension and mitral valve prolapse) were recorded on the web-based data recruitment forms. Between February 2011 and October 2011, we conducted a prospective community-based, case–control study of polycystic kidney disease in Erciyes University, which is located in Kayseri, Turkey. A total of 28 normotensive ADPKD patients with normal renal function included to our study. Based on these, we recruited a matched healthy subjects (n = 30) who were admitted to our family medicine center for routine check-up. They had no known diseases and were not currently taking any drugs.
The estimated glomerular filtration rate (eGFR) level was calculated using the Modification of Diet in Renal Disease (MDRD) formula: MDRD=186× (serum creatinine [mg/dl])−1.154×age−0.203. A correction factor of 0.742 was used for women (Citation8). According to this calculation, subjects with renal dysfunction and undergoing hemodialysis/peritoneal dialysis were excluded. The study was approved by the university ethics committee and local hospital review committee. All participants provided written informed consent.
Age, gender and biochemical measurements, such as fasting blood glucose, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglyceride and serum creatinine levels, were recorded.
All participants underwent 12-lead electrocardiography (ECG) at 25 mm/s (paper speed) and transthoracic echocardiography by Vivid 7 instruments (GE Medical Systems, Milwaukee, WI, USA), with a 2.5-MHz transducer. The LV ejection fraction (LVEF) was calculated using the formula:
LVEF% = (LVEDV − LVESV)/LVEDV × 100
where LVEDV is the LV end-diastolic volume and LVESV is the LV end-systolic volume.
The LV mass (LVM) was calculated using the formula (Citation9):
LVM = 0.8 × (1.04 [(IVSd + LVEDD + LPWd)3 − (LVEDD)3 + 0.6 g.
where IVSd is the interventricular septum diameter, LVEDD is the LV end-diastolic diameter and LPWd is the LV posterior wall thickness.
The healthy group had no organ system or cardiovascular disease on physical, electrocardiographic and echocardiographic examination.
Study population
The following participants were excluded from analysis: (i) those under 17 years and above 60 years; (ii) those with a history of myocardial infarction, ischemic heart disease, LVEF lower than 50%, severe valvular regurtation, moderate or severe valvular stenosis, cardiomyopathy, cardiac arrhythmia and stroke; (iii) those with known high systemic blood pressure (SBP ≥ 140 mmHg or diastolic blood pressure, DBP, ≥ 90 mmHg) (Citation10) and using antihypertensive drugs; (iv) those with < 10 g/dl hemoglobin; (v) those with diseases interfering with the autonomic nervous system, including diabetes mellitus, liver diseases, Parkinson's disease, porphyria and amyloidosis; (vi) those with neurological diseases; (vii) chronic obstructive pulmonary disease; and (viii) smokers. However, we excluded the patients with renal dysfunction (eGFR < 60 ml/min) in our study.
Exercise testing
All patients in the study underwent exercise treadmill testing using the Bruce protocol. Predicted peak heart rate was calculated as (220 − age) and the aim was to reach at least 85% of the age- predicted heart rates. The end of exercise was flagged, and at least 5 min of post-exercise heart rate recorded with the subject at rest. Qualified exercise physiologists and/or cardiology fellows prospectively collected physiologic and hemodynamic data during testing, including symptoms, heart rate, heart rhythm, blood pressure (BP) and estimated functional capacity in metabolic equivalents (METs, where 1 MET = 3.5 ml/kg per min of oxygen consumption). HRR indices were defined as the reduction in heart rate from the rate at peak exercise to the rate at the 1st, 2nd, 3rd and 5th minutes after the cessation of the exercise stress test; these results were noted as HRR1, HRR2, HRR3 and HRR5, respectively.
Diagnostic criteria for hypertension
Hypertension was considered present if the SBP was > 140 mmHg and/or DBP was > 90 mmHg in two or more BP measurements (Citation10). Ambulatory monitoring was performed to all individuals by using Mobil-0-Graph recorder.
Statistical analysis
All analyses were performed using SPSS V 16.0 for Windows (version 16.0, SPSS, Chicago, IL, USA). Quantitative variables were expressed as mean value ± SD for parametric variables and median and minimum-maximum levels for non-parametric variables. Comparisons of parametric values between the two groups were performed by means of independent-samples t-test. Comparisons of non-parametric values between the two groups were performed by Mann–Whitney U test. Categorical variables were compared by the chi-squared test. A two-tailed p-value < 0.05 was considered statistically significant.
Results
The study population included 28 normotensive ADPKD patients (12 male, mean age: 42.4 ± 10.9 years) with normal renal function and 30 healthy subjects as controls patients (10 male, mean age: 47.2 ± 12.4 years). The baseline characteristics of the study groups are shown in . The ADPKD patients and healthy subjects were similar with respect to age, gender distribution, fasting glucose and cholesterol levels according to the basic clinical and demographic features. All subjects were normotensive, and the SBP and DBP and resting heart rate were higher in healthy subjects compared with patients with ADPKD. Initially, all participants had normal 12-lead ECG results and sinus rhythm. All completed the exercise stress test without rhythm abnormalities, ischemic changes, or other complications.
Table I. Demographic and clinical futures of the patients and the controls.
LVEDD, LV end-systolic diameter, LVPWd, LVEF, left atrial size, systolic pulmonary artery pressure and right ventricular end-diastolic diameter were similar in patients with ADPKD and the control group (). Although no difference was established in IVSd, LVM was higher in control subjects compared with patients in the ADPKD group (10.7±1.4 vs 10.0± 1.3, p=0.16; 171.2±29.1 vs 155.7±33.8, p=0.12).
Table II. Comparison of echocardiographic and exercise stress test derived variables of patients and controls (mean±SD).
Comparisons of the maximal heart rate, maximal SBP and DBP, exercise duration, and metabolic equivalents made using during the exercise stress test values between ADPKD group and healthy subjects are also summarized in . We have measured ambulatory BP measurements (ABPM) to all participants (). The ADPKD patients and healthy subjects were normotensive and similar with respect to ABPM (p>0.05). The HRR indices of the patients with ADPKD and the control group are shown in . According to these results, the 1st- and 2nd-minute HRR indices of patients with ADPKD were significantly lower than those of the healthy control group (27.1±7.9 vs 32.0±7.9; p=0.023 and 46.9±11.5 vs 53.0±9.0; p=0.029, respectively). Similarly, HRR indices after the 3rd and 5th minutes of the recovery period were significantly lower in patients with ADPKD when compared with those indices in the control group (56.7±12.0 vs 65.1±11.2; p=0.008 and 62.5±13.8 vs 76.6±15.5; p=0.001, respectively). On the other hand, no significant differences were observed in metabolic equivalents (p = 0.40), maximal heart rate (p = 0.16), maximal SBP (p = 0.74) and DBP (p = 0.23) achieved during the exercise stress test between the two groups.
Figure 1. The heart rate recovery (HRR) indices study participants in autosomal-dominant polycystic kidney disease (ADPKD) and control groups.
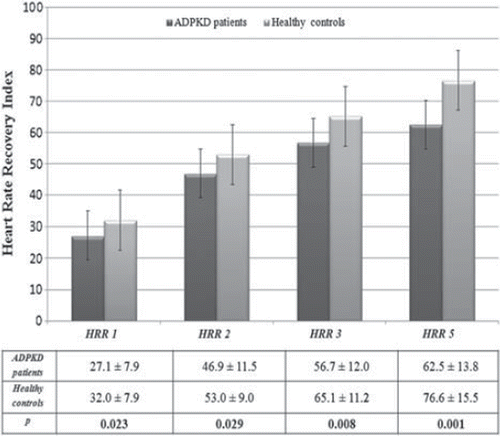
Table III. Data from ambulatory blood pressure measurements of the study subjects.
Discussion
In this study, we showed that HRR indices were impaired in the 1st, 2nd, 3rd and 5th minutes of the recovery period after maximal exercise testing in normotensive ADPKD patients who had normal renal functions compared with healthy control subjects. To the best of our knowledge, this is the first study to show an impaired HRR index among normotensive early-stage ADPKD patients.
The autonomic nervous system plays a major role in the control of cardiovascular function regulation. It has been shown that through stimulation of the sympathetic nervous system and simultaneous regression of the parasympathetic nervous system, the heart rate rises during exercise (Citation11). Conversely, when parasympathetic reactivation occurs together with sympathetic inhibition, the heart rate decreases immediately after exercise (Citation12). In the first study about HRR in 1982, Savin et al. (Citation13) showed that sympathetic withdrawal contributes more to HRR soon after peak exercise cessation, while at lower rates parasympathetic activation plays a greater role. HRR has been researched in several diseases such as, obstructive sleep apnea syndrome, diabetes mellitus, chronic heart failure, coronary heart disease, familial Mediterranean fever, systemic lupus erythematosus, sarcoidosis and Behcet's disease (Citation3,Citation14,Citation15).
Several studies have shown that impairment in the HRR index present with abnormal autonomic control of the heart is a powerful independent predictor of cardiovascular and all-cause mortality in healthy individuals (Citation11,Citation16). Jouven et al. (Citation17) reported that in healthy subjects with a resting heart rate was more than 75 beats/min increased a relative risk of 3.92, in subjects with an increase in heart rate during exercise that was less than 89 beats/min has relative risk of 6.18 and a decrease in heart rate of less than 25 beats/min after the termination of exercise increased has a relative risk of 2.20 for sudden cardiac death (Citation17).
Previous studies have shown that cardiovascular mortality is among the most important causes of death in patients with ADPKD (Citation18,Citation19). A post-mortem study by Iglesias et al. (Citation19) showed that the most common cause of death was cardiovascular in these patients. Fick et al. (Citation18) demonstrated that mortality due to cardiac disease was the most common cause of death (36%) in 129 subjects with APKD. The early appearance and long duration of hypertension occurring after LVH are important contributors to cardiac disease. However, Fick et al. (Citation18) showed that the cardiac mortality rate was similar in subjects with or without end-stage renal disease. LVH is a cardiac reaction to hypertension and is an important known cardiovascular risk factor (Citation20). The importance of this is that it leads to deterioration of cardiac tissue, which begins before impairment of renal functions. We also observed in our study that IVSd and LVM were higher in control subjects compared with the ADPKD group but insignificantly.
Hypertension is very common an early finding in patients with ADPKD that occurs in 50–62% of patients when renal function is still normal. When renal function has become impaired, almost all of the patients have hypertension (Citation21). Renal structural remodeling and vascular alteration play an important role in the pathogenesis of hypertension. Because of the pressure of cysts, renal structural vascularization decreases and this leads to the development of renal ischemia. A large amount of avascular renal area has been shown by renal angiography in hypertensive ADPKD patients (Citation22).
A result of renal ischemia causes activation of the renin system. In fact, it has long been known that hypertensive patients with ADPKD may have an increased blood renin level and activated RAAS (renin–angiotensin–aldosterone system) (Citation23). Although there have been discussions about blood renin levels in these patients, both increased intrarenal and circulating RAAS activity have been shown in patients with ADPKD (Citation24). As a result, renal ischemia and activated RAAS can also lead to sympathetic activation in patients with ADPKD (Citation25). It is well recognized that RAAS is a strong activator of the sympathetic nervous system (Citation26). Klein et al. (Citation6) showed that muscle sympathetic nerve activity was increased in hypertensive ADPKD patients. Another study published by Iversen et al. (Citation27) demonstrated raised sympathetic hyperactivity in hypertensive ADPKD patients. Another study published by Cerasola et al. (Citation5) examined sympathetic activity in hypertensive ADPKD patients and patients with essential hypertension. They showed that plasma catecholamine levels were higher in hypertensive ADPKD patients without renal failure than in essential hypertensive patients.
As a result of consulting these studies, we were interested in examining the HRR index in early-stage normotensive ADPKD patients. Despite the fact that some studies have demonstrated the presence of sympathetic activation in hypertensive patients with ADPKD (Citation5,Citation6), there are no studies in the literature about autonomic dysfunction in normotensive ADPKD patients using the HRR index. Herein we showed that autonomic dysfunction can occur in ADPKD patients in the absence of hypertension and LVH.
Study limitations
The limitations of the present study are the relatively small number of patients and the results are based on a single center. Also, the plasma renin activity ratios were not measured. The other limitation is that this study is of a cross-sectional design. Patients with ADPKD could not be observed prospectively for cardiovascular events and mortality. For this reasons, long-term follow-up and large-scale prospective studies are required to establish the predictive value of HRR for the future development of cardiovascular events and mortality in patients with ADPKD.
Conclusion
Our findings suggest that impairment in the HRR index is associated with normotensive early-stage ADPKD patients. Probably, increased renal ischemia and activation of RAAS contribute to impairment in the autonomic nervous system in these patients before the development of hypertension. Even if ADPKD patients are normotensive, there appears to be an association with autonomic dysfunction and polycystic kidney disease. Therefore, physicians should be alert in terms of cardiovascular events.
Conflict of interest: None.
References
- La Rovere MT, Bigger JT, Jr., Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351: 478–484.
- Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol. 2007;34:362–368.
- Ardic I, Kaya MG, Yarlioglues M, Dogdu O, Celikbilek M, Akpek M, . Assessment of heart rate recovery index in patients with familial Mediterranean fever. Rheumatol Int. 2011;31:121–125.
- Mancia G, Giannattasio C, Failla M, Sega R, Parati G. Systolic blood pressure and pulse pressure: Role of 24-h mean values and variability in the determination of organ damage. J Hypertens Suppl. 1999;17:55–61.
- Cerasola G, Vecchi M, Mule G, Cottone S, Mangano MT, Andronico G, . Sympathetic activity and blood pressure pattern in autosomal dominant polycystic kidney disease hypertensives. Am J Nephrol. 1998;18:391–398.
- Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001;12:2427–2433.
- Johansson M, Gao SA, Friberg P, Annerstedt M, Bergstrom G, Carlstrom J, . Reduced baroreflex effectiveness index in hypertensive patients with chronic renal failure. Am J Hypertens. 2005;18:995–1000; discussion 16.
- Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, . Recommendations for improving serum creatinine measurement: A report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18.
- Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618.
- Joint National Committee. The seventh report of the Joint National Committee on detection, evaluation and treatment of high blood pressure (JNC 7). J Am Med Assoc. 2003; 289:2560–2572.
- Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357.
- Imai K, Sato H, Hori M, Kusuoka H, Ozaki H, Yokoyama H, . Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J Am Coll Cardiol. 1994;24: 1529–1535.
- Savin WM, Davidson DM, Haskell WL. Autonomic contribution to heart rate recovery from exercise in humans. J Appl Physiol. 1982;53:1572–1575.
- Dogdu O, Yarlioglues M, Kaya MG, Ardic I, Oguzhan N, Akpek M, . Deterioration of heart rate recovery index in patients with systemic lupus erythematosus. J Rheumatol. 2010;37:2511–2515.
- Ardic I, Kaya MG, Yarlioglues M, Dogdu O, Buyukoglan H, Kalay N, . Impaired heart rate recovery index in patients with sarcoidosis. Chest. 2011;139:60–68.
- Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398.
- Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med. 2005;352:1951–1958.
- Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048–2056.
- Iglesias CG, Torres VE, Offord KP, Holley KE, Beard CM, Kurland LT. Epidemiology of adult polycystic kidney disease, Olmsted County, Minnesota: 1935–1980. Am J Kidney Dis. 1983;2:630–639.
- Verdecchia P, Schillaci G, Guerrieri M, Gatteschi C, Benemio G, Boldrini F, . Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536.
- Valvo E, Gammaro L, Tessitore N, Panzetta G, Lupo A, Loschiavo C, . Hypertension of polycystic kidney disease: Mechanisms and hemodynamic alterations. Am J Nephrol. 1985;5:176–181.
- Cornell SH. Angiography in polycystic disease of the kidneys. J Urol. 1970;103:24–26.
- Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: Early occurrence and unique aspects. J Am Soc Nephrol. 2001;12:194–200.
- Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323: 1091–1096.
- DiBona GF. The kidney in the pathogenesis of hypertension: The role of renal nerves. Am J Kidney Dis. 1985;5: A27–A31.
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197.
- Iversen J FH, Norgaard N, Strandgaard S. Sympathetic nervous activity in adult polycystic kidney disease [Abstract]. Hypertension. 1996;28.