Abstract
To explore association of hypertension with coexistence of inflammation and endothelial dysfunction and abnormal metabolism, a community-based study was conducted among Mongolian people in China. Demographic characteristics and lifestyle risk factors were investigated, blood pressure, body weight and waist circumference were measured, fasting blood samples were obtained to measure blood lipids, fasting plasma glucose and the biomarkers of inflammation and endothelial dysfunction, C-reactive protein (CRP), soluble intercellular cell adhesion molecule-1 (sICAM-1), soluble E-selectin (sE-selectin) and angiotensin II. Rates of abnormal metabolism, elevated CRP, elevated sICAM-1, elevated sE-selectin and elevated angiotensin II as well as coexistence of abnormal metabolism with the elevated biomarkers were all higher in hypertensives than these in normotensives (all p < 0.01). Compared with subjects with normal metabolism and without any elevated biomarker, multivariate adjusted odds ratio (95% confidence interval) of hypertension associated with abnormal metabolism, elevated CRP, elevated sICAM-1, elevated sE-selectin, elevated angiotensin II, coexistences of abnormal metabolism with elevated CRP, elevated sICAM-1,elevated sE-selectin and elevated angiotensin II were 2.209 (1.594–3.062), 2.820 (1.992–3.992), 2.370 (1.665–3.374), 1.893 (1.331–2.691), 2.545 (1.793–3.612), 2.990 (2.102–4.252), 2.551 (1.775–3.667), 2.223 (1.544–3.220), 3.135 (2.185–4.519), respectively. In conclusion, this study indicated that inflammation and endothelial dysfunction was associated with hypertension and abnormal metabolism, and individuals with co-existence of abnormal metabolism with inflammation and endothelial dysfunction had higher risk of prevalent hypertension among Mongolian population. This study suggests that further study on treatment for hypertension patients with coexistence of abnormal metabolism with inflammation and endothelial dysfunction should be conducted in the near future.
Introduction
In recent decades, some studies showed that chronic inflammation and endothelial dysfunction were associated with hypertension (Citation1–4), and other studies demonstrated that chronic metabolic abnormality, including abdominal obesity (Citation5–7), lipid abnormality (Citation6,Citation8–10) and diabetes (Citation11), was also associated with hypertension. Some studies have further shown that inflammation and endothelial dysfunction are not only associated with atherosclerosis, cardiovascular disease and hypertension, but also with metabolic syndrome (MetS), a condition of multiple metabolic abnormality including obesity or abdominal obesity, high blood pressure (BP), hyperlipidemia, abnormal glucose metabolism (Citation12), increasing the risk of type 2 diabetes and cardiovascular disease (Citation13). For example, the biomarkers of inflammation and endothelial dysfunction such as C-reactive protein (CRP), interleukin-6, soluble intercellular cell adhesion molecule-1 (sICAM-1), soluble vascular cell adhesion molecule-1 and soluble E-selectin (sE-selectin) have been reported to be associated with chronic metabolic abnormality (Citation14–16). Therefore, it is possible that there is a co-effect between chronic inflammation and endothelial dysfunction and chronic metabolic abnormality on hypertension. However, few studies focused on co-effect of inflammation and endothelial dysfunction with chronic metabolic abnormality on hypertension in an Asian population, especially in Mongolian population, a minority living in north China who maintained a traditional lifestyle. In the present study, we examined the effects between coexistence of some components of abnormal metabolism with elevated inflammation and endothelial dysfunction biomarkers including CRP, sICAM-1, sE-selectin and angiotensin II on hypertension in a Mongolian population from Inner Mongolia, China.
Methods
Study subjects
A community-based, cross-sectional study on hypertension was conducted between 2002 and 2003 in Inner Mongolia, China. The methods for the study participant selection and data collection are presented in detail elsewhere (Citation17). Briefly, all local residents aged 20 years and older were recruited from 32 villages in two adjacent townships located in Kezuohou Banner (county) and Naiman Banner. Most participants were Mongolian who maintained a traditional diet that was high in fat and salt. Among the eligible 3475 residents, 2589 individuals signed informed consent forms and participated in the study. This study was approved by the Soochow University ethics committee.
Data collection and examination
A standard questionnaire was administered by trained staff to obtain data on demographic characteristics, lifestyle risk factors and family history of hypertension. Three BP measurements were taken for every participant using a standard mercury sphygmomanometer according to a standard study protocol after the subject had been resting for 30 min. The mean of the three BP measurements was used for the analysis.
Body weight, height and waist circumference were measured with a balance beam scale after subjects removed their shoes and were wearing light clothing. Waist circumference was measured at the level of 1 cm above the umbilicus. Body mass index (BMI) was calculated as weight in kilogram divided by the square of the height in meters.
Overnight fasting blood samples were drawn by venipuncture from all subjects in the morning after at least 8 h of fasting, and fasting plasma glucose (FPG) was examined using a glucose meter (Roche, Basel, Switzerland) in the field. Serum was subsequently isolated from whole blood, and all serum samples were frozen at − 80°C.
Concentration of triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C) were assessed enzymatically on a Beckman Synchrony CX5 Delta Clinical System (Beckman Coulter, Inc., Fullerton, CA, USA) using commercial reagents. Concentration of CRP was determined by an immunoturbidimetric assay on a Beckman Synchron CX5 Delta Clinical System using commercial reagents. sICAM-1 and sE-selectin were measured by an ELISA assay (R&D SysteMetS, Minneapolis, MN, USA) employing the quantitative sandwich enzyme immunoassay technique. Concentration of angiotensin II was measured by radioimmunoassay following separation by high-performance liquid chromatography.
Cigarette smoking was defined as having smoked at least 1 cigarette per day for 1 year or more. Heavy alcohol drinking was defined as consuming at least 50 g of alcohol per day for 1 year or more. Hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg and/or use of antihypertensive medication in the 2 weeks prior to being examined. MetS was defined according to the modified Adult Treatment Panel III (ATP III) recommendations for Asian Americans (Citation18). Individuals were considered to have MetS if they had three or more of the following risk factors: WC ≥ 90 cm for men and WC ≥ 80 cm for women; TG ≥ 1.70 mmol/l, or specific treatment for this lipid abnormality; HDL-C < 1.04 mmol/l in men and < 1.30 mmol/l in women, or specific treatment for this lipid abnormality; BP ≥ 130/85 mmHg, or treatment of previously diagnosed hypertension; or FPG ≥ 5.6 mmol/l, or previously diagnosed type 2 diabetes in the present study. Abnormal metabolism was defined as having components of MetS except for high BP, namely abdominal obesity, TG abnormality, HDL-C abnormality and FPG abnormality. Elevated biomarkers of inflammation and endothelial dysfunction were defined as the following according to the upper quartile of the biomarkers: elevated CRP, elevated sICAM-1, elevated sE-selectin and elevated angiotensin II were defined as CRP > 11.40 mg/l, sICAM-1 > 395.2 ng/ml, sE-selectin > 24.80 ng/ml and angiotensin II > 71.30 pg/ml, respectively.
Statistical analysis
Demographic characteristics, levels of metabolic indices and biomarkers of inflammation and endothelial dysfunction were presented with median or rate (percentage), and were compared between hypertensives and normotensive, and between abnormal metabolism and normal metabolism groups with Mann–Whitney U test or χ2 test. Rates of elevated biomarkers of inflammation and endothelial dysfunction and abnormal metabolism were calculated by BP status and compared between hypertensives and normotensives with χ2 test. Levels of biomarkers were described with median by BP status and number of abnormal metabolic indices; and association between levels of biomarkers and number of abnormal metabolic indices was analyzed with the Spearman correlation. Associations of hypertension with abnormal metabolism, elevated biomarkers and coexistence of abnormal metabolism with elevated biomarkers were analyzed using a logistic regression model. Odds ratio (OR) and 95% confident interval (CI) were calculated, respectively. All p-values were two-tailed and statistical significance was defined as p ≤ 0.05. Statistical analyses were conducted using SAS 9.2 (SAS Institute Inc, Cary, North Carolina).
Result
General characteristics of the subjects stratified by metabolic status and BP status are presented in . Under stratification by metabolic status, hypertensives were more likely to have older age, high rates of male, smoking and heavy drinking, greater waist circumference, high levels of TG, FPG and CRP compared with normotensives in both normal metabolism, and abnormal metabolism stratifications; hypertensives were more likely to have great BMI, high sICAM-1, sE-selectin and angiotensin II levels compared with normotensives in abnormal metabolism stratification (all p <0.05) but not in normal metabolism stratification (p> 0.05). Under stratification by BP status, there was no difference in age between subjects with normal metabolism and those with abnormal metabolism in both hypertension and normotension stratifications (p > 0.05); subjects with abnormal metabolism were more likely to have low rates of male, smoking and heavy drinking, greater BMI and waist circumference, high levels of TG, FPG, CRP, sICAM-1 and sE-selectin, low levels of HDL-C and angiotensin II, compared with those with normal metabolism in both hypertension and normotension stratifications (all p < 0.05).
Table I. General characteristics of 2589 subjects by blood pressure status and metabolism status.
Compared with normotensives, hypertensives had higher rates of abnormal metabolism, elevated CRP, elevated sICAM-1, elevated sE-selectin and elevated angiotensin II (all p < 0.01). Furthermore, the rates of coexistence of abnormal metabolism with elevated CRP, elevated sICAM-1, elevated sE-selectin and elevated angiotensin II were also all higher in hypertensives compared with those in normotensives (all p < 0.01) ().
Table II. Rates of abnormal metabolism, elevated biomarkers of inflammation and coexistence of abnormal metabolism and elevated biomarkers of inflammation by blood pressure status.
Levels of CRP, sICAM-1, sE-selectin and angiotensin II in subjects by BP status and number of abnormal metabolic indices are illustrated in . Except for angiotensin II, levels of CRP, sICAM-1 and sE-selectin all increased with number of abnormal metabolic indices both in hypertensives and normotensives (all p < 0.001).
Figure 1. Levels of C-reactive protein (CRP), soluble intercellular cell adhesion molecule-1 (sICAM-1), E-selectin and angiotensin II (Ang II) by number of abnormal metabolic indices and hypertension status.
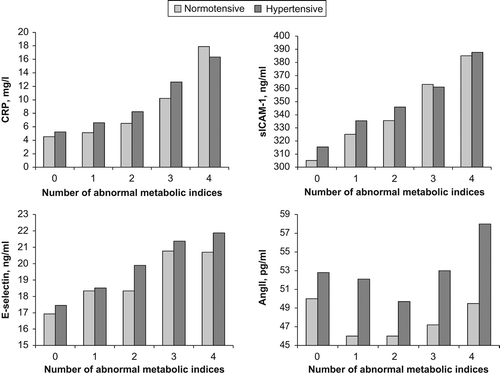
There were significant associations between abnormal metabolism, elevated inflammation biomarkers and the coexistence of abnormal metabolism, with the elevated inflammation and endothelial dysfunction biomarkers and hypertension. Compared with subjects without both abnormal metabolism and any of elevated inflammation and endothelial dysfunction biomarkers, unadjusted ORs of hypertension associated with abnormal metabolism, elevated CRP, elevated sICAM-1, elevated sE-selectin, elevated angiotensin II, and the coexistences of abnormal metabolism with the elevated CRP, elevated sICAM-1, elevated sE-selectin, elevated angiotensin II were 1.615, 2.861, 2.202, 1.848, 2.341, 3.027, 2.294, 2.077 and 2.785, respectively. The ORs remained significant after adjusted for multivariate ().
Table III. Odds ratios (OR) and 95% confidence intervals (95% CI) of hypertension associated with abnormal metabolism, elevated biomarker of inflammation and their coexistence.
Discussion
In this cross-sectional study among a Mongolian population, we found that increased levels of inflammation and endothelial dysfunction biomarkers such as CRP, sICAM-1, sE-selectin and angiotensin II and their coexistences with abnormal metabolism including abdominal obesity, TG abnormality, HDL-C abnormality and FPG abnormality were positively and significantly associated with hypertension. Individuals with higher levels of CRP, sICAM-1, sE-selectin and angiotensin II and their coexistences with abnormal metabolism were all at an increased risk of hypertension compared with those with lower levels of the biomarkers and without abnormal metabolism. In addition, our findings showed not only a trend of increasing of level of the biomarkers with number of abnormal metabolism indexes, but also a trend that coexistences of the biomarkers with abnormal metabolism increased risk of hypertension. These data provide further support that inflammation and endothelial dysfunction, abnormal metabolism as well as coexistence of abnormal metabolism with inflammation and endothelial dysfunction are associated with hypertension.
Our study contributes to understanding of association among abnormal metabolism, inflammation and endothelial dysfunction and hypertension. Firstly, abnormal metabolism was associated with biomarkers of inflammation and endothelial dysfunction independently of BP status. Secondly, coexistence of abnormal metabolism with elevated biomarker of inflammation and endothelial dysfunction probably increased risk of hypertension. Our study shows results similar to that of some studies which inflammation and endothelial dysfunction are not only associated with hypertension, but also with MetS (Citation12,Citation13).
In this study, we analyzed association of hypertension with abnormal metabolism, biomarkers of inflammation and endothelial dysfunction, and coexistence of abnormal metabolism with biomarkers of inflammation and endothelial dysfunction among a large Mongolian population, and angiotensin II was included as a biomarker of inflammation and endothelial function. To a certain degree, the biomarkers such as CRP, sICAM-1 and sE-selectin reflect either the status of inflammation or the status of endothelial dysfunction (Citation19–21). Aside from the role of a vasoconstrictor, angiotensin II also induces endothelial dysfunction and inflammation resulting in accelerated progression of atherosclerosis (Citation22,Citation23).
Some previous studies have documented that inflammation biomarkers might be an important precursor to cardiovascular disease including hypertension (Citation24,Citation25), MetS and type 2 diabetes (Citation26,Citation27). For example, Sung and colleagues reported that increased CRP was an independent risk factor of hypertension (Citation2). Haffner et al. (Citation28,Citation29) provided accumulating evidences that inflammation was an important risk factor of cardiovascular disease; their studies demonstrated that elevated CRP was associated with increased risk of cardiovascular disease and diabetes mellitus, and, accordingly, they believed that a proinflammatory state should be served as one of components of MetS. Festa et al. (Citation30) and Kraja et al. (Citation31) also reported that elevated CRP and elevated sICAM-1 as well as elevated sE-selectin were all associated with components of MetS, inflammation and endothelial dysfunction might induce insulin resistance (Citation32); insulin resistance is a key link of MetS. There were inverse results that insulin resistance may in turn exacerbate inflammation by increasing cytokine and adipo-chemokine expression (including TNF-α, IL-6, leptin and others), elevating free fatty acid levels, and impairing endothelial nitric oxide synthase activity (Citation33,Citation34). Both inflammation and MetS or insulin resistance are two important links in the mechanism of hypertension. Systematic inflammation promotes the development of hypertension by inducing insulin resistance or abnormal metabolism, or insulin resistance promotes the development of hypertension by exacerbating the inflammation reaction. In our study, there was a close relation among abnormal metabolism, inflammation and endothelial dysfunction and hypertension. Our findings support that inflammation and endothelial dysfunction are associated with hypertension and MetS, and that coexistence of abnormal metabolism with inflammation and endothelial dysfunction increase risk of hypertension. Our study might provide a clue for clinical treatment and risk evaluation for hypertension, though it was a community-based study. It is necessary to further study whether the coexistence of abnormal metabolism with inflammation and endothelial dysfunction affect the effectiveness of treatment for hypertension and promote incidence of other cardiovascular diseases.
Limitations should be mentioned in the present study. First, this was a cross-sectional study, therefore, a causal relationship between inflammation and endothelial dysfunction and co-existence of abnormal metabolism with inflammation and endothelial dysfunction and hypertension could not be established, cohort studies are warranted to further evaluate the causal relationship. Secondly, approximately 25% of individuals who were eligible for inclusion did not participate in the study, which would unavoidably cause some selection bias. However, we believe this bias is minimal because it is unlikely that participants chose not to participate due to their BP or biomarker levels, which they did not know. Furthermore, several important biomarkers of inflammation and endothelial dysfunction, such as tumor necrosis factor-alpha (TNF-a), IL-6, von Willebrand factor and VCAM-1, were not measured in our study. Data on endothelium independent flow-mediated vasodilation were also not collected.
In summary, our study indicated that inflammation and endothelial dysfunction was associated with hypertension and abnormal metabolism, and individuals with co-existence of abnormal metabolism with inflammation and endothelial dysfunction had higher risk of prevalent hypertension among the Mongolian population. This study suggests that further study on treatment for hypertension patients with coexistence of abnormal metabolism with inflammation and endothelial dysfunction should be conducted in future.
Sources of funding
The National Natural Science Foundation of China (Grant No. 81172761) and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Acknowledgements
We are deeply appreciative of the participants who are actively engaging in the study, and thank the Kezuohouqi Banner Center for Disease Prevention and Control, and the Naiman Banner Center for Disease Prevention and Control for their support and assistance.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by National Natural Science Foundation of China (Grant No. 81172761) and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
References
- Bautista LE, Lopez-Jaramillo P, Vera LM, Casas JP, Otero AP, Guaracao AI. Is C-reactive protein an independent risk factor for essential hypertension? J Hypertens. 2001;19:857–861.
- Sung KC, Suh JY, Kim BS, Kang JH, Kim H, Lee MH, et al. High sensitivity C-reactive protein as an independent risk factor for essential hypertension. Am J Hypertens. 2003;16:429–433.
- Gupta V, Sachdeva S, Khan AS, Haque SF. Endothelial dysfunction and inflammation in different stages of essential hypertension. Saudi J Kidney Dis Transpl. 2011;22:97–103.
- Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132–140.
- Chandrasekaran N, Amalraj E, Datta M, Krishnamurthy PV, Sankaran JR, Rajasambandam P. Association between obesity and hypertension in south Indian patients. Indian Heart J. 1994;46:21–24.
- Zhang M, Batu B, Tong W, Liu YY, Liu Y, Zhang Y. Clustering of hyperlipidemia, hyperglycemia, alcohol drinking, overweight and central obesity and hypertension in Mongolian people, China. CVD Prev Control. 2009;4:163–169.
- Gus M, Fuchs SC, Moreira LB, Moraes RS, Wiehe M, Silva AF, et al. Association between different measurements of obesity and the incidence of hypertension. Am J Hypertens. 2004;17:50–53.
- Guo ZR, Hu XS, Wu M, Zhou MH, Zhou ZY. [A prospective study on the association between dyslipidemia and hypertension]. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30: 554–558.
- Bonaa KH, Thelle DS. Association between blood pressure and serum lipids in a population. The Tromso Study. Circulation. 1991;83:1305–1314.
- Sesso HD, Buring JE, Chown MJ, Ridker PM, Gaziano JM. A prospective study of plasma lipid levels and hypertension in women. Arch Intern Med. 2005;165:2420–2427.
- Movahed MR, Sattur S, Hashemzadeh M. Independent association between type 2 diabetes mellitus and hypertension over a period of 10 years in a large inpatient population. Clin Exp Hypertens. 2010;32:198–201.
- Miyao M, Furuta M, Kondo T, Sakakibara H, Ishihara S, Yamanaka K, Yamada S. The relationship of high-density lipoprotein cholesterol to obesity, drinking and smoking habits. Nagoya J Med Sci 1993;55:65–70.
- Pacholczyk M, Ferenc T, Kowalski J. The metabolic syndrome. Part I: Definitions and diagnostic criteria for its identification. Epidemiology and relationship with cardiovascular and type 2 diabetes risk. Postepy Hig Med Dosw (Online) 2008;62:530–542.
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: Associations with obesity, insulin resistance, and endothelial dysfunction: A potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978.
- Leinonen E, Hurt-Camejo E, Wiklund O, Hulten LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387–394.
- Hossain M, Faruque MO, Kabir G, Hassan N, Sikdar D, Nahar Q, et al. Association of serum TNF-α and IL-6 with insulin secretion and insulin resistance in IFG and IGT subjects in a Bangladeshi population. Int J Diabetes Mellitus. 2010;2:165–168.
- Zhang M, Batu B, Tong W, Li H, Lin Z, Li Y, et al. Prehypertension and cardiovascular risk factor clustering among Mongolian population in rural and animal husbandry area, Inner Mongolia, China. Circ J. 2009;73:1437–1441.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752.
- Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406.
- Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–1923.
- Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342: 836–843.
- Schiffrin EL, Touyz RM. Multiple actions of angiotensin II in hypertension: Benefits of AT1 receptor blockade. J Am Coll Cardiol. 2003;42:911–913.
- Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–1652.
- Anuurad E, Tracy RP, Pearson TA, Kim K, Berglund L. Synergistic role of inflammation and insulin resistance as coronary artery disease risk factors in African Americans and Caucasians. Atherosclerosis. 2009;205:290–295.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: Moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138.
- Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM, et al. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study.[see comment]. Diabetes Care. 2002;25:2016–2021.
- Sattar N, Gaw A, Scherbakova O, Ford I, O’Reilly DS, Haffner SM, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108:414–419.
- Haffner SM. The metabolic syndrome: Inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97: 3A–11A.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497.
- Festa A, D’Agostino R, Jr., Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42–47.
- Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, Hopkins PN, et al. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr Metab (Lond). 2007;4:28.
- Park K, Steffes M, Lee DH, Himes JH, Jacobs DR, Jr.Association of inflammation with worsening HOMA-insulin resistance. Diabetologia. 2009;52:2337–2344.
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801.
- Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279–288.