Abstract
This study has been designed to evaluate the impact of adiponectin levels on left ventricular geometry and function in visceral obesity-associated hypertension. 94 consecutive subjects, 53 of them were hypertensives and 41 normotensives with age ≤ 65 years, subgrouped according to the presence or absence of visceral obesity, were studied. Total adiponectin levels were measured by a validated competitive radioimmunoassay. Left ventricular telediastolic internal diameter, interventricular septum, posterior wall thickness, total left ventricular mass (LVM) and normalized for height to the 2.7 power (LVM/h2.7), relative wall thickness, left ventricular ejection fraction by echocardiography and isovolumic relaxation time, E/A ratio and deceleration time of E velocity, by pulsed-wave Doppler, were calculated. Plasma adiponectin levels were significantly lower in visceral obesity-associated hypertensives than lean hypertensives (p < 0.001) and in lean normotensives (p < 0.001). LVM and LVM/h2.7 were significantly (p < 0.05) higher in both hypertensive groups, and in visceral obesity-associated normotensives in comparison with lean normotensives. Adiponectin levels correlated inversely with LVM/h2.7 but only in normotensives (adjusted R squared 0.77, p < 0.0001) and hypertensives (0.67, p < 0.0001) subjects with visceral obesity. Multiple regression analysis indicated that adiponectin levels remain significantly associated (p < 0.001) to LVM/h2.7 also when adjusted for age, gender, body mass index, waist to hip ratio and mean blood pressure. Our data suggest an important role of adiponectin in increased LVM/h2.7 in visceral obesity-associated normotensive and hypertensive subjects. In this last group, adiponectin, more than blood pressure, may be able to explain the development of cardiac damage.
Introduction
Cardiovascular protection is one of the most important goals of antihypertensive treatment (Citation1). On the other hand, obesity, especially when characterized by visceral fat distribution, is often associated with hypertension and overall mortality (Citation2). In fact, for many years, it has been well demonstrated that obese patients with visceral fat distribution are more likely to be hypertensive than lean subjects, and that weight gain is predictive of earlier onset of hypertension. In addition, the association of visceral obesity and hypertension seems to confer a higher cardiovascular risk than that detectable in lean hypertensives (Citation2–4). This association is characterized by adverse morphologic and functional changes in the cardiovascular system. They include an increased left ventricular mass (LVM) and early left ventricular (LV) dysfunction (Citation5,Citation6). Although changes in the heart caused by hypertension and/or visceral obesity are well known, the effective mechanisms are not. Despite largely emphasized hemodynamic effects and growth factors, other metabolic and inflammatory factors exist. In view of this, an increase in LVM might occur independently of blood pressure (Citation7). Accordingly, we have previously recognized some clinical predictors of LV hypertrophy (LVH) in obese hypertensive patients, such as the concomitant presence of a positive family history of both hypertension and visceral obesity (Citation8) and an overproduction of transforming growth factor beta2 (Citation9).
In addition, it is now known that adipocytes are active endocrine and paracrine cells secreting an increasing number of mediators called “adipocitokynes”, including adiponectin, a collagen-like protein abundantly produced in adipose tissue that plays an important role in the modulation of glucose and lipid metabolism (Citation10,Citation11). It has been demonstrated that adiponectin has several antiatherogenic and antidiabetic properties. Lower levels of adiponectin are associated with higher risk of myocardial infarction (Citation12), with increased carotid atherosclerosis (Citation13) and with vulnerability of coronary plaque (Citation14). Lower levels of adiponectin have been also reported in some conditions associated with insulin resistance, such as visceral obesity, hypertension and diabetes (Citation10,Citation11,Citation15,Citation16).
Receptors of adiponectin are expressed in cultured cardiac myocytes and heart tissue, and experimental evidences suggest that adiponectin inhibits hypertrophic signaling in the myocardium and may thus influence cardiac remodeling (Citation10,Citation11,Citation17–19).
Despite these data, the role of adiponectin on cardiac changes occurring in visceral obesity-associated hypertension has not been investigated.
The present study has been designed to evaluate the impact of adiponectin levels on LV geometry and function in visceral obesity-associated hypertension. Accordingly, plasma levels of adiponectin and their relationships with measurements of LV geometry and function have been analyzed in normotensive and hypertensive both lean and visceral obese subjects. The main goal of the study was to evaluate the role of adiponectin in the cardiac changes occurring in both hypertensive and normotensive visceral obese subjects.
Subjects and methods
Subjects
Subjects eligible for the study were screened at the center of hypertension and metabolic disease at the Department of Internal Medicine, University of Palermo (Italy). The study population consisted of 94 consecutive subjects; 53 of them were hypertensives and 41 normotensives with age ≤ 65 years. Each patient gave a written consent after received a detailed description of study procedure. The study was approved by ethics committee of our institution. All participants underwent a standardized examination that included interviews, anthropometry, BP measurements, resting electrocardiogram, Doppler echocardiography and a fasting blood draw.
Information regarding medical history, drug use, alcohol and cigarette consumption was collected during a face-to-face interview using a standardized questionnaire. Height was measured on a clinic stadiometer. Body weight was measured by electrical bioimpedance using a body composition analyzer model TB-300 (Tanita, Tokyo, Japan). Body mass index (BMI) was calculated. Waist circumference was measured by plastic tape as the narrowest circumference between the lower rib margin and anterior superior iliac crest. Brachial BP was measured three times during three different visits, with a standard sphygmomanometer after 5 min of rest. The mean value was used in statistical analysis.
The subjects were defined as obese on the basis of sex-specific 85th percentile of BMI values, as reported in the Italian Consensus Conference on Obesity (Citation20). Accordingly, the men with BMI higher than or equal to 30 kg/m2 and the women with BMI higher than or equal to 27.3 kg/m2 were considered obese. Conversely, the men with BMI less than or equal to 25 kg/m2 and the women with BMI less than or equal to 24.7 kg/m2 were considered lean. Visceral fat distribution was defined on the basis of sex-specific 85th percentile of waist to hip ratio (WHR). The cut-off values of visceral obesity were considered 0.81 for women and 0.92 for men (Citation21).
Subjects under antihypertensive treatment or with a casual blood pressure (SBP) ≥ 140 mmHg and/or with casual diastolic blood pressure (DBP) ≥ 90 mmHg were considered hypertensives. Arterial blood pressure was measured with an appropriate large cuff in obese subjects. Systolic (SBP), diastolic (DBP) and mean blood pressure (MBP) were determined. MBP was calculated by the sum of DBP plus one third of pulse pressure. All the hypertensives were untreated for at least 3 weeks before the study. Lean normotensives were volunteer subjects by us recruited to undergo a clinical check-up and found to be healthy.
Exclusion criteria included severe hypertension, cardiovascular diseases, renal failure, insulin-dependent or -independent diabetes mellitus, hyperlipoproteinemia, electrolyte imbalance, smoking habit and alcoholism, or psychiatric problems.
All the subjects included in this study were subdivided as follows:
(1) Lean normotensives: this group consisted of 15 subjects (six females and nine males, mean age 46.5 ± 13.3 years);
(2) Visceral obese normotensives: this group consisted of 26 subjects (12 females and 14 males; mean age 52.1 ± 8.4 years);
(3) Lean hypertensives: this group consisted of 17 subjects (eight females and nine males; mean age 49.2 ± 7.5 years);
(4) Visceral obese hypertensives: this group consisted of 36 subjects (16 females and 20 males; mean age 51.0 ± 9.2 years).
Biochemical measurements
Patients underwent a general analytical laboratory parameters profile including BUN, creatinine and clearance, glycemia, electrolytes and cholesterol, by routine laboratory methods.
Peripheral venous blood was obtained from each patient and the sera were isolated and stored at − 70°C. Total adiponectin levels were measured by a validated competitive radioimmunoassay (Linco Research Inc., St Charles, MO) with a coefficient of variation of 3.4%. In a previous analysis, adiponectin levels had excellent intraclass correlation coefficient measured in participants over a period of 1 year and were not substantially affected by transport conditions (Citation22).
Echocardiographic measurements
All patients underwent an echocardiography examination M and B-mode, by a computerized echocardiography (ESAOTE, Italy) for the determination of following parameters: LV telediastolic internal diameter (LVIDd), interventricular septum (IVSTd), and posterior wall thickness (PWTd). The Penn convention was used to calculate LVM. LVM was normalized for height to the 2.7 power (LVM/h2.7) (Citation7,Citation23). Accordingly, all the hypertensives with LVM/h2.7 ≥ 50 g/m2.7 for men and ≥ 47 g/m2.7 for women were considered to have LVH. The relative wall thickness (RWT) by formula [(PWTd/LVIDd)× 2] was also calculated. Ejection fraction from LV end-diastolic and end-systolic volumes was measured from the apical four-chamber view, using the ellipsoidal single-plane algorithm. Mean ejection fraction was automatically calculated by the echocardiographic processing system. In our laboratory, the ejection fraction calculated over five consecutive beats permitted optimal reproducibility and accuracy (Citation23).
LV relaxation and filling were evaluated by pulsed-wave Doppler interrogation of the LV inflow tract from the apical four-chamber view, with the sample volume placed at the tips of the mitral valve. After a stable signal of the transmitral flow velocity was obtained, the Doppler cursor was moved toward the LV outflow tract in the apical five-chamber view for recording both mitral and aortic signals, including the closing click of the aortic valve and the opening click of the mitral valve. Doppler signals were recorded at high speed (80–120 mm/s) with the subjects in held expiration. An average of five beats was used for analysis.
Isovolumic relaxation time (IVRT) was calculated as the time from the closure click of the aortic valve to the opening click of the mitral valve. When either the closing or opening click was not identified, the time from the end of the aortic flow to the onset of mitral flow from the continuous wave interrogation of the LV inflow–outflow tract was used. Peak early transmitral flow velocity (E), peak late transmitral flow velocity (A) and the deceleration time of E velocity (DTE) were measured at the tips of mitral leaflets at the maximum amplitude of E velocity. DTE was measured as the time from peak E velocity to the time when the E wave descent intercepts the zero line.
Statistical analysis
Data are shown as mean± SD: the chi-square and the Fisher exact test were used for contingency table analysis. To explore for statistical significant differences, one-way ANOVA was performed with Bonferroni connection post hoc analysis for comparison between groups. Multivariate linear regression analysis was performed to study relationship among independent variables and LVM/h2.7. The best prediction model was build after a bivariate analysis. A p-value of 0.25 was used as cut-off for variable inclusion into the model. The final model was build with a backward selection of variables until the p-value of each variable into the model was inferior to 0.25. We used a bootstrap procedure (50 replications per time) to validate the regression model and compute standard errors. Multiple fractional polynomial analysis was performed to study the best power fit between each independent variable and the dependent ones. Linear regression lines and their confidence intervals were computed by conventional methods for visual representations. STAT/SE, version 9.2 for Windows (StataCorp. College Station, Texas), was used to both analyze and graph the data.
Results
Characteristics of lean and visceral obese groups
All the groups were comparable with regard to sex and age. Lean and visceral obese normotensives and hypertensives were also comparable with regard to systolic, diastolic and mean blood pressure. Both lean and visceral obese groups were also comparable accordingly to BMI and WHR values. Plasma adiponectin levels were significantly lower in visceral obese hypertensives than those detectable both in lean hypertensives (p < 0.001) and in lean normotensives (p < 0.001). In addition, adiponectin levels were lower but not significantly, both in visceral obese hypertensives respect to visceral obese normotensives and in lean hypertensives in comparison with lean normotensives ().
Table I. Clinical characteristics and adiponectin levels in normotensive and hypertensive lean and visceral obese subjects.
No significant difference in adiponectin levels were detected in all the groups subdivided according to gender ().
Table II. Plasma adiponectin levels in all the groups subdivided according to gender.a
Echocardiographic measurements
LVM and LVM/h2.7 values were significantly (p < 0.05) higher in visceral obese hypertensives, in lean hypertensives and in visceral obese normotensives in comparison with lean normotensives. No significant changes in remain cardiac measurements among the groups were found ().
Table III. Echocardiographic measurements in normotensive and hypertensive lean and visceral obese subjects.
Correlations
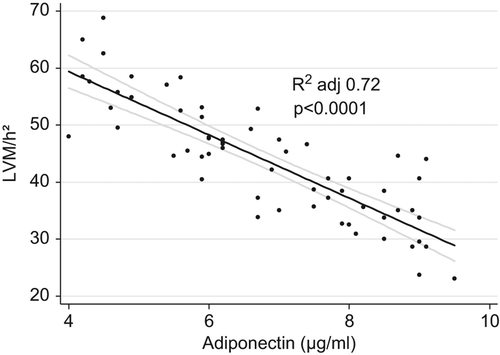
Linear regression analysis demonstrated that adiponectin levels correlated inversely with LVM/h2.7 but only in normotensive (adjusted R squared 0.77; p < 0.0001) and hypertensive visceral obese participants (adjusted R squared 0.67; p < 0.0001) (). This relationship remained significant (adjusted R squared 0.72; p < 0.0001) even when visceral obese normotensives and hypertensives were analyzed together ().
Figure 1. Correlation between plasma adiponectin levels and indexed left ventricular mass in all the groups studied.
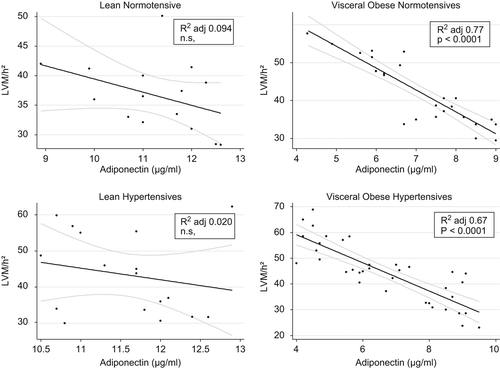
Figure 2. Correlation between plasma adiponectin levels and indexed left ventricular mass in both groups of obese subjects.
In all the subjects studied, multiple regression analysis indicated that adiponectin levels remained significantly (p < 0.001) associated with LVM/h2.7 also when adjusted for age, gender, BMI, WHR and MBP ().
Table IV. Multiple regression analysis.
Discussion and conclusions
To our knowledge, this is the first study able to indicate the relevant role of adiponectin to explain the changes in cardiac geometry in visceral obese subjects both hypertensive and normotensive. In fact, the main data of our study suggest that visceral obese subjects both normotensive and hypertensive had lower serum adiponectin levels and higher levels of LVM/h2.7, than those detectable respectively in lean normotensive and in lean hypertensive subjects. In addition, systolic and diastolic function seems to be preserved in all the groups. Furthermore, data from multiple regression analysis indicated a significant inverse relationship between LVM/h2.7 and serum adiponectin levels but only in both visceral obese groups. This association was independent of age, gender, BMI and MBP. This finding appears interesting, indicating that adiponectin might be considered an important factor associated with changes in cardiac geometry in normotensive and hypertensive visceral obese subjects. Adiponectin levels seem to be more important than blood pressure in the recognizing of LVH in visceral obese hypertensives. In our opinion, the lack of abnormalities in diastolic function might be explained by the age of patients studied. Accordingly, the increased values of indexed LVM by us found might be considered an early cardiac alteration in these subjects.
The relation between hypoadiponectinemia and the risk of hypertension is well known (Citation24). Several cross-sectional studies had previous shown an inverse relation between adiponectin levels and MBP (Citation25,Citation26). Recently, Chow et al. (Citation27) added another important observation to the relationship between adipose tissue and high BP, by demonstrating, for the first time, an inverse relation between plasma adiponectin concentration and the future development of hypertension. In this study, a low serum adiponectin level at baseline was a powerful predictor of future hypertension. Moreover, hypoadiponectinemia has been reported to contribute to the development of obesity-related hypertension at least in part directly, in addition to its effects through insulin resistance (Citation28).
On the other hand, it is known that LVH is very frequent in obese subjects (Citation3,Citation4,Citation29). The increased metabolic needs accompanying obesity cause hyperdynamic circulation as the blood volume increases. Additionally, peripheral vascular resistance and increased vascular stiffness are developed, leading to hemodynamic overload. Consequently, an increase in LVM may be expected (Citation2,Citation3,Citation30). However, the common belief is that the influential mechanisms are not limited to hemodynamic changes. In fact, a large population-based study has shown that only about 50% of LVM variation can be explained by demographic and hemodynamic factors (Citation31). Thus, non-hemodynamic mechanisms are likely to contribute to increase in LVM and wall thickness (WT). In this field, an important role has been recently attributed to visceral obesity (Citation2,Citation3,Citation8,Citation32).
The negative relation between adiponectin and LVM/h2.7 found by us in normotensive and hypertensive visceral obese subjects may also contribute to this mechanism. In other words, in these subjects a lack of protective effects of adiponectin may cause an increase in LVM/h2.7. Our data are in agreement with results from previous studies indicating that serum adiponectin is inversely and independently associated also with electrocardiographically diagnosed LVH (Citation19), and that adiponectin is able to influence cardiac remodeling (Citation33). It has been shown experimentally that decreased plasma adiponectin levels may lead to LVH by directly affecting LVM, and that an increase in adiponectin levels may be effective in correcting the pathologic change in cardiac structure (Citation18). Accordingly, adiponectin administration might have a practical clinical application to restore LVH (Citation34).
In our study, hypertension might be considered a confounding factor, since it could cause both LVH and may be associated with reduction in adiponectin levels. For these reasons, we have studied lean and visceral obese subjects, both normotensive and hypertensive. Results from our multiple regression analysis indicated an independent association between low adiponectin levels and higher LVM/h2.7 values, which might contribute to clarify this question. Accordingly, in our opinion, it is possible to suggest that when we study the relationships between adipocytokines and hypertension, we must simultaneously evaluate the presence or absence of visceral obesity.
Several mechanisms have been suggested to explain the hypothesis that hypoadiponectinemia may cause LVH. A possible effect of adiponectin on LVM may be to inhibit hypertrophic signaling directly in the myocardium by activating adenosine monophosphate-activated kinase, which activates eukaryotic elongation factor-2 kinase and the inhibitor of cardiac myocyte protein synthesis (Citation18,Citation19).
Another possible mechanism may be the suppression of angiotensin II-stimulated myocyte hypertrophy with adiponectin (Citation35), but our study is not sufficient to explain these mechanisms because of its cross-section design. However, the presence of a negative relationship between adiponectin and LVM/h2.7 also in visceral obesity without hypertension might suggest that decreased myocardial protein synthesis via the inhibition of hypertrophic signaling may play a more important role in explaining adiponectin-associated myocardial protection.
There are some limitations to our study. In fact, it has been designed to be a cross-sectional study. Evaluation of the cause-effect relationship between hypoadiponectinemia and LVH would require a prospective study design with a cohort base and larger numbers of cases. Therefore, we cannot prove causality or predictive ability, but only discern association.
In conclusion, the present study indicates that decreased adiponectin plasma levels were associated with increased LVM/h2.7 values in normotensive and hypertensive visceral obese subjects. In this particular subgroup of hypertensives, characterized by the contemporaneous presence of visceral obesity, adiponectin, more than blood pressure, is able to explain the development of cardiac damage. Accordingly, also considering that the protective role of adiponectin in CVD is still controversial (Citation36), clinical long-term follow-up with a large number of participants is needed to validate this hypothesis, evaluating whether the increase in adiponectin levels induced by antihypertensive therapy might be able to improve LV geometry. Accordingly, in this particular subgroup of hypertensive subjects with visceral obesity, adiponectin levels might become a new target of antihypertensive treatment.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- The Task force for the management of arterial hypertension of the European Society of Hypertension (ESC) and of the European Society of Cardiology (ESC). 2007 ESH/ESC guidelines for the management of arterial hypertension. Eur Heart J. 2007;28:1462–1536.
- Nguyen T, Lau DCW. The obesity epidemic and its impact on hypertension. Canad J Cardiol. 2012;28:326–333.
- Licata G, Scaglione R, Barbagallo M, Parrinello G, Capuana G, Lipari R, et al. Effect of obesity on left ventricular function studied by radionuclide angiocardiography. Int J Obesity. 1991;15:295–302.
- Licata G, Scaglione R, Capuana G, Parrinello G, Di Vincenzo D, Mazzola G. Hypertension in obese subjects: A distinct hypertensive subgroup. J Hum Hypertens. 1990; 4:37–41.
- Scaglione R, Di Chiara MA, Indovina A, Lipari R, Ganguzza A, Parrinello G, et al. Left ventricular diastolic and systolic function in normotensive obese subjects: Influence of degree and duration of obesity. Eur Heart J. 1992;13: 738–742.
- Licata G, Scaglione R, Ganguzza A, Corrao S, Donatelli M, Parrinello G, et al. Central obesity and hypertension: Relationship between fasting serum insulin, plasma renin activity and diastolic blood pressure in young obese subjects. Am J Hypertens. 1994;7:314–320.
- De Simone G, Kizer JR, Chinali M, Roman MJ, Bella JN, Best LG, et al. Normalization for body size and population-attributable risk of left ventricular hypertrophy: The Strong Heart Study. Am J Hypertens. 2005;18:191–196.
- Licata G, Scaglione R, Corrao S, Ganguzza A, Mazzola G, Arnone S, et al. Heredity and obesity-associated hypertension: Impact on hormonal characteristics and left ventricular mass. J Hypertens. 1995, 13:611–618.
- Parrinello G, Licata A, Colomba D, Di Chiara T, Argano C, Bologna P, et al. Left ventricular filling abnormalities and obesity associated hypertension: Relationship with overproduction of circulating transforming growth factor beta1. J Hum Hypertens. 2005;19:543–550.
- Scaglione R, Di Chiara T, Cariello T, Licata G. Visceral obesity and metabolic syndrome: Two faces of the same medal?Intern Emerg Med. 2010;5:111–119.
- Nishida M, Funahashi T, Shimomura I. Pathophysiological significance of adiponectin. Med Mol Morphol. 2007; 40:55–67.
- Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:134–141.
- Kojima S, Funahashi T, Maruyoshi H, Honda O, Sugiyama S, Kavano H, et al. Levels of the adipocyte-derived plasma protein, adiponectin, have a close relationship with ateroma. Thromb Res. 2005;115:483–490.
- Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533.
- Matsuzawa Y. The metabolic syndrome and adipocytokines. FEBS. 2006;580:2917–2921.
- Diez JJ, Iglesias P. The role of novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol. 2003;148:293–300.
- Di Chiara T, Argano C, Corrao S, Scaglione R, Licata G. Hypoadiponectinemia: A link between visceral obesity and metabolic syndrome. J Nutr Metab. 2012; ID175245:1–7.
- Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10: 1384–1389.
- Mitsuhashi H, Yatsuya H, Tamakowshi K, Matsushita K, Otsuka R, Wada K, et al. Adiponectin level and left ventricular hypertrophy in Japanese men. Hypertension. 2007;49: 1448–1454.
- Crepaldi G, Belfiore F, Bosello O, Caviezel F, Contaldo F, Enzi G, Melchionda N. Special report: Italian Consensus Conference-Overweight, Obesity and Health. Int J Obesity. 1991;15:781–790.
- Scaglione R, Ganguzza A, Corrao S, Parrinello G, Merlino G, Dichiara MA, et al. Central obesity and hypertension: Pathophysiologic role of renal haemodynamics and function. Int J Obesity. 1995;19:403–409.
- Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: Stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–652.
- Corrao S, Parrinello G, Arnone S, Indovina A, Scaglione R, Licata G. Influence of obesity on the echocardiographic evaluation of left ventricular ejection fraction by area- length method: Comparison with radionuclide angiography. J Cardiovasc Diagn Proced. 1993;11:127–134.
- Schillaci G, Pirro M. Hypoadiponectinemia: A novel link between obesity and hypertension?Hypertension. 2007;49: 1217–1229.
- Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003; 16:72–75.
- Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43: 1318–1323.
- Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, et.al. Hypoadiponectinemia as a predictor for the development of hypertension: A 5-year prospective study. Hypertension. 2007;49:1455–1461.
- Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity- related hypertension. Hypertension. 2006;47:1108–1116.
- Shunkert H. Obesity and target organ damage: The heart. Int J Obesity. 2002;26 Suppl 4:S15–S20.
- Vasan RS. Cardiac function and obesity. Heart. 2003;89: 1127–1129.
- Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: The Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006; 48:2285–2292.
- Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004; 110:3081–3087.
- Ybarra J, Resmini E, Planas F, Navarro-Lopez F, Webb S, Pou JM, et al. Relationship between adiponectin and left atrium size in uncomplicated obese patients: Adiponectin, a link between fat and heart. Obes Surg. 2009;19:1324–1332.
- Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: Disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39.
- Hong SJ, Park CG, Seo HS, Oh DJ, Ro YM. Associations among plasma adiponectin, hypertension, left ventricular diastolic function and left ventricular mass index. Blood Press. 2004;13:236–242.
- Lindberg S, Mogelvang R, Pedersen SH, Bjerre M, Frystyk J, Flyvbjerg A et al. Relation of serum adiponectin levels to number of traditional atherosclerotic risk factors and all-cause mortality and major adverse cardiovascular events (from the Copenhagen City Heart Study). Am J Cardiol. 2013;111:1139–1145.
