Abstract
The glicyrrhizic acid, contained in licorice, has a mineralcorticoid-like effect. Chronic excess intake of licorice induces the rare syndrome of “apparent mineralcorticoid excess”, due to the inhibitory effect of glicyrrhizic acid on 11 β-hydroxysteroid dehydrogenase type 2 determining clinical/biochemical manifestations as resistant hypertension, metabolic alkalosis and severe hypokalemia. We report a typical clinical case of licorice abuse to emphasize the importance of a detailed anamnesis, which is essential for the diagnosis, avoid unnecessary and expensive investigations, and reduce the duration of hospitalization. We also provide an appraisal of the pathophysiology of “apparent mineralcorticoid excess” syndrome, still an often forgotten or unrecognized cause of hypokalemia and hypertension.
Introduction
Licorice abuse is an often forgotten or unrecognized cause of hypokalemic muscular weakness with rhabdomyolisis and hypertension that induces an acquired clinical syndrome also called “apparent excess of mineralcorticoid (AME)” syndrome. The AME syndrome is characterized by a defective activation of 11 β-hydroxysteroid dehydrogenase type 2 (11βHSD2), which converts cortisol to cortisone. The consequent cortisol excess stimulates the mineralocorticoid receptors in the renal distal tubules and collecting ducts resulting in excessive sodium reabsorption, potassium wasting, metabolic alkalosis, potentially life-threatening arrhythmia, hypertension, low plasma renin activity (PRA) and aldosterone levels (Citation1–4). If recognized early, patients may recover without clinical sequelae.
Case report
A 72-year-old man presented at the Emergency Department (ED) complaining of severe and progressive muscular weakness, cramped pain in both legs and peripheral bilateral edema. He denied chest pain, shortness of breath, orthopnea or decreased urinary output, fever, nausea and vomiting, and diarrhea in the last days. Biochemical analyses revealed severe hypokalemia (1.9 mmol/l). His medical history included hypertension, insulin- dependent type 2 diabetes mellitus and chronic renal failure [eGFR (MDRD): 34 ml/min/1.73 m2], no family history of cardiovascular disease and no alcohol abuse. Hypertension has been present in his clinical history for 20 years and was never investigated in order to rule out the presence of a secondary form of hypertension. The patient has been under antihypertensive medicaments for 10 years and his treatment at the admission was based on rampril 5 mg/day, hydrochlorothiazide 25 mg/day and amlodipine 10 mg/day. The biochemical evidence of renal failure was first documented about 5 years ago with both diabetic nephropathy and nephroangiosclerosis likely contributing to its etiology. Physical examination showed peripheral bilateral edema and swollen hands. Neurological evaluation revealed symmetrical flaccid paralysis with hyporeflexia in all extremities. Blood pressure was scarcely controlled, which was confirmed at admission (165/70 mmHg).
The patient was then sent to our clinic where blood tests confirmed a severe hypokalemia (2.1 mmol/l) and increased rhabdomyolysis indexes (myoglobin: 3525 μg/l, creatine phosphokinase 2921 IU/l), metabolic alkalosis (pH 7.59, HCO3− 40.6 mmol/l), myoglobinuria (199 μg/l), low levels of 24-h urinary potassium (< 5 mmol) and 24-h urinary sodium was 240 mmol. Electrocardiography showed U-wave, wide T-wave and apparent prolongation of QTc, known signs of hypokalemia.
The most common forms of hypokalemia such as diuretics abuse and insulin overdose, intake of β2-adrenergic and α-antagonist drugs, vomiting and diarrhea, alcohol abuse, Cushing's syndrome and diabetic ketoacidosis were ruled out by clinical and biochemical evaluation. PRA and plasma aldosterone concentration were low both at baseline and after captopril test (50 mg of captopril) PRA: 0.2 μg/l/h and 0.3 μg/l/h; plasma aldosterone: 97 and 105 pmol/l respectively (normal range: PRA: 0.2–3.30 μg/l/h, aldosterone: 20–415 pmol/l), which ruled out primary or secondary hyperaldosteronism.
Serum potassium gradually normalized after suspension of hydrochlorothiazide, upon potassium canreonate (100 mg/day) and intravenous potassium supplementation (80 mmol of KCl). The symptoms improved, including edema and cramping pains, and the indexes of rhabdomyolysis declined.
In consideration of the prompt resolution of the clinical and biochemical picture, the patient was carefully reconsidered regarding his anamnesis, which was essential for the diagnosis revealing the consumption of about 2 ounces of pure licorice per week for a month as a means to quit smoking.
Six months after discharge and suspension of licorice, the patient was asymptomatic with no sign of edema, no complains of cramping pains and normal neurological evaluation. Blood pressure was 132/74 mmHg with antihypertensive treatment based on ramipril 5 mg/day and amlodipine 10 mg/day. Laboratory tests showed plasma K in the normal range (4.1 mmol/l) without K supplements, myoglobin 78 μg/l, creatine phosphokinase 125 IU/l, plasma pH 7.41 and HCO3− 24.2 mmol/l. Renal function remained stable: eGFR 35 ml/min/1.73 m2.
Discussion
Licorice abuse represents a rare and often forgotten or unrecognized form of hypokalemia, associated with arterial hypertension. Most common symptoms are muscle weakness or paralysis, and cardiac arrhythmias associated with peripheral edema, related to an increased sodium reabsorption.
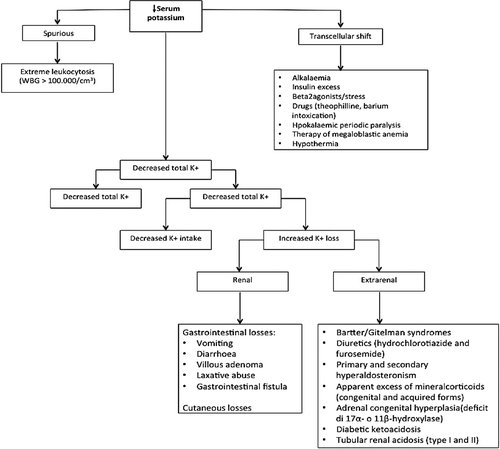
In healthy individuals, the absorption of dietary potassium (about 100 mmol/day) occurs in the stomach and proximal small intestine; about 10 mmol/l are eliminated in the intestine and through sweating, the remaining 90% is redistributed through short- and long-term mechanisms. The first is represented by the intra-extracellular shift, which includes the amount of potassium that passes to the extracellular space under the influence of various substances and hormones such as insulin, β2 agonist and α-antagonist drugs, and phosphodiesterase inhibitors that determine the potassium's transition into the cells through the direct or indirect activation of sodium–potassium ATPase. The long-term mechanism is represented by the renal potassium handling, which is modulated by potassium intake, hormones, acid-base factors and sodium delivery to the distal nephron. The filtered potassium is reabsorbed by proximal tubule and thick ascending limb of Henle's loop. Distal tubules and collecting ducts are responsible for potassium excretion (Citation5).
summarizes the main causes of hypokalemia. In our patient, most of the causes of hypokalemia were excluded by anamnestic and biochemical investigations, while the resolution of symptoms, prompt normalization of serum potassium and progressive decline of rhabdomyolysis indexes led us to suspect a form of abuse of licorice-induced hypokalemia.
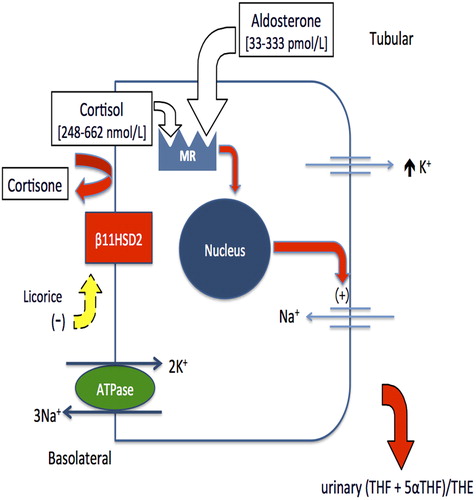
Glycyrrhizic acid, which is the active component of licorice, causes, when in excess, a metabolic syndrome characterized by hypertension, metabolic alkalosis and hypokalemia – the AME syndrome (Citation1–4). Glycyrrhizic acid, in fact, inhibits the 11β HSD2, an enzyme that converts cortisol to its inactive metabolite cortisone (Citation6). It is predominantly expressed, together with the mineralocorticoid receptors (MRs), in the renal distal tubules and collecting ducts but also in the placenta where it protects the fetus from an excessive amount of maternal cortisol (Citation7). Cortisol is a potent agonist of the epithelial type 1 MRs, and thus reduced activity or total deficiency of 11βHSD2 exposes the kidney to cortisol excess, which causes excess mineralocorticoid activity (Citation6) (). MRs have, in fact, the same affinity for cortisol and aldosterone, and the inactivation of cortisol to cortisone by 11βHSD2 prevents MRs being activated by cortisol (Citation1–4,Citation6).
The form of hypokalemia in our patient belongs to a large group of diseases characterized by defective 11βHSD2: the AME syndrome (Citation1,Citation2). Clinical features characterizing AME syndrome, which suggest an excess of a mineralocorticoid-like substance, are hypertension, plasma volume expansion, hypokalemic alkalosis and a suppressed renin–angiotensin–aldosterone system. There are congenital or acquired forms of AME syndrome (Citation1–4). The first is an autosomal recessive genetic disorder that results in a juvenile and severe hypertensive syndrome, with low birth weight, failure to thrive and persistent polydipsia and polyuria. Biochemical profiles show severe hypokalemia, metabolic alkalosis, low plasma aldosterone and suppressed PRA, this latter suggesting volume expansion, which explains the elevated blood pressure. Biochemical diagnosis of AME syndrome is based on the evidence of the increased urinary cortisol metabolites to cortisone ratio, which is measured by the sum of the urinary tetrahydrocortisol (THF) and allotetrahydrocortisol (5αΤHF), divided by the concentration of tetrahydrocortisone (THE): THF + 5αΤΗF/THE.
A variant of this form, the so-called “type II AME” has been documented in several patients and is characterized by a milder phenotype, with onset in the late adolescence or early adulthood and only a mildly deranged urinary THF + 5αTHF/THE ratio (Citation2).
The same pathophysiology is shared by the acquired forms of AME. Patients consuming excessive quantities of licorice, carbenoxolone or flavonoids present with hypertension and hypokalemia, which may be severe enough to cause dangerous cardiac arrhythmias. Both PRA and aldosterone levels are suppressed or reduced, and exchangeable sodium is increased as well as urinary THF + 5αTHF/THE ratio (Citation5). The acquired forms of AME are often responsive to antialdosterone drugs and are reversible upon stopping the ingestion of these substances.
Conclusion
This case report has the aim to underline the need of a careful history taking for a condition where it is not only essential for the diagnosis but it may also be a determinant for sparing the patient from expensive and unnecessary investigations, such as cerebral computed tomography or cerebral/spinal nuclear magnetic resonance, electromyography and neurologic evaluation. Thus, it is very important for the physicians to keep in mind licorice abuse as a cause of hypertension, hypokalemic muscular weakness and rhabdomyolisis, which is still an often forgotten or unrecognized cause of hypokalemia.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Palermo M, Quinkler M, Stewart PM. Apparent mineralocorticoid excess syndrome: An overview. Arq Bras Endocrinol Metabol. 2004;48:687–696.
- Mantero F, Palermo M, Petrelli MD, Tedde R, Stewart PM, Shackleton CHL. Apparent mineralocorticoid excess: Type I and type II. Steroids. 1996;61:193–196.
- Stewart PM. Mineralocorticoid hypertension. Lancet. 1999; 353:1341–1347.
- Melander O. Genetic factor in hypertension – What is known and what does it mean?. Blood Press. 2001;10:254–270.
- Giebisch GH, Wang WH. Potassium transport – An update. J Nephrol. 2010;23 (Suppl 16):S97–104.
- Armanini D, Lewicka S, Pratesi C, Scali M, Zennaro M, Zovato S, et al. Further studies on the mechanism of the mineralocorticoid action of licorice in humans. J Endocrinol Invest. 1996;19:624–629.
- Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11 β-hydroxysteroid dehydrogenase type 2 enzyme. Mol Cell Endocrinol. 1994; 105:R11–R17.